A chemoenzymatic reduction of conjugated C=C double bonds has been developed by Diederik J. Opperman, Frank Hollmann and co-workers, University of the Free State, South Africa, and Delft University of Technology, The Netherlands, respectively.
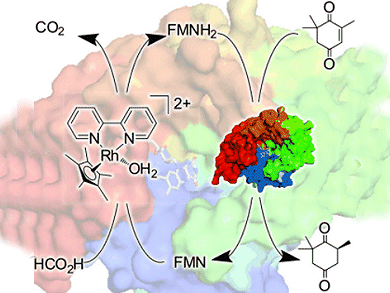
Chiral carbonyl compounds can be prepared by the enoate reductase (ER)-catalyzed asymmetric reduction of conjugated C=C double bonds. Conventional processes use the expensive nicotinamide cofactor [NAD(P)H] to regenerate the primary reductant, which necessitates an additional recycling strategy to ensure cost-effective use of the cofactor.
Based on the fact that the nicotinamide does not play a direct role in the catalytic mechanism, the group have developed NAD(P)-independent regeneration of ERs by using the rhodium catalyst [Cp*Rh(bpy)(H2O)]2+. This catalyst, with good activity and stability at high temperature, is an excellent candidate for thermophilic oxidoreductions. The robust and readily available ER homologue chromate reductase (CrS) was used as a model enzyme in the reduction of conjugated C=C double bonds.
The combination of CrS and [Cp*Rh(bpy)(H2O)]2+ provides a system much simpler than the classical ones and shows promising catalytic performance. Optimization and upscaling of the chemoenzymatic reduction system are currently underway.
Image: © Wiley-VCH
- Chemoenzymatic Reduction of Conjugated C=C Double Bonds,
J. Bernard, E. van Heerden, I. W. C. E. Arends, D. J. Opperman, F. Hollmann,
ChemCatChem 2012, 4, 196–199.
DOI: 10.1002/cctc.201100312