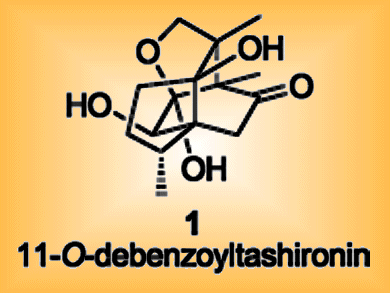
During development, neurons assemble into functional networks by growing out axons and dendrites — collectively called neurites — which connect synaptically to other neurons. Khusiol is a sesquiterpenoid natural product with a tricyclic core. It shares its 3,6,7,7-tetramethyloctahydro-3a,6a-ethanoindene framework with 11-O-debenzoyltashironin, a natural product that promotes neurite outgrowth.

Starting from an enantiomerically enriched cis-1,2-dihydrocatechol, Martin Banwell and co-workers, Australian National University, Canberra, devised a 16-step route to khusiol. Construction of the khusiol framework hinged on an intramolecular Diels–Alder reaction. The synthesis was completed by introducing a key methyl group by conjugate addition of the organocopper species generated in situ from methyl magnesium bromide and CuBr•SMe2 to an intermediate enone.
This work should enable easier access to the parent compound 11-O-debenzoyltashironin and its congeners, allowing structure–activity studies of this biologically important class of compounds.
- Approaches to the Neurotrophically Active Natural Product 11-O-Debenzoyltashironin: A Chemoenzymatic Total Synthesis of the Structurally Related Sesquiterpene Khusiol,
M. K. Sharma, M. G. Banwell, A. C. Willis, A. D. Rae,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201100913