Organocatalysts are non-toxic and working at air- and water-tolerating reaction conditions with simple reaction set-ups. Organocatalytic methods often require catalyst loadings as high as 30 mol% for the achievement of high conversions in reasonable reaction times. Immobilization of organocatalysts represents an attractive technique, which allows simple recovery and reuse of the catalysts leading to a substantial reduction of the effective catalyst loading. Additionally, the immobilization of organocatalysts onto solid supports allows a simplified workup by direct filtration of the catalytic species. Because of their stability and recyclability, solid supported organocatalysts are particularly suitable for continuous flow systems, which allow large-scale synthesis usually accompanied by higher turnover numbers (TONs).
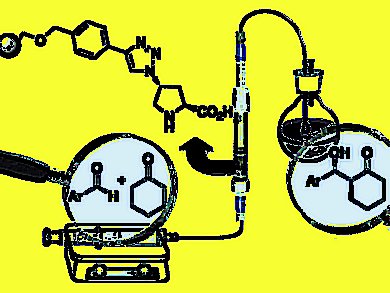
Miquel A. Pericàs, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues present the preparation of a novel, polystyrenesupported proline derivative as a highly active and stereoselective catalyst for aldol reactions, and its use for the implementation of a single-pass continuous flow process for the production of diastereo- and enantiomerically pure aldol products. Up to four different enantiomerically pure aldol products can be sequentially produced in gram amounts with the same catalyst sample.
The catalyst can be recovered by simple filtration and shows very high reusability.
The effective catalyst loading could be reduced to 1.6 %; a six-fold reduction of catalyst loading compared to the corresponding batch process.
- A Solid-Supported Organocatalyst for Continuous-Flow Enantioselective Aldol Reactions,
Carles Ayats, Andrea H. Henseler, Miquel A. Pericàs,
ChemSusChem 2012.
DOI: 10.1002/cssc.201100570

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

