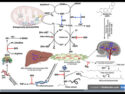
Biosynthesis of Methyl Chavicol (Estragole)
Ethereal basil oil derived from the Reunion chemotype contains methyl chavicol [(3), estragole] as its chief component, a compound present only to a very limited extent in oil from the Mediterranean chemotype. Methyl chavicol is also a C10 compound, but it is not a terpene, since its skeleton is derived not from two isoprene units, but rather from the amino acid phenylalanine (11) [12]. The first reaction step in its formation is a cleavage of ammonia with the aid of the enzyme phenylalanine ammonia lyase (PAL).
.gif)
Figure 6. Biosynthesis of methyl chavicol (3).
The cinnamic acid that results is next hydroxylated at the para position — by molecular oxygen in the presence of cytochrome P-450 — and subsequently methylated. Reduction of the cinnamyl alcohol can lead to two isomeric compounds, anethole (13) and the target methyl chavicol (3).
Anethole is an important substance in aniseed, star anise, and fennel, whereas methyl chavicol occurs above all in basil of the Reunion type, as well as in tarragon.
Reference
[12] S. A. Goff et al., Science 2006, 311, 815–819. DOI: 10.1126/science.1112614