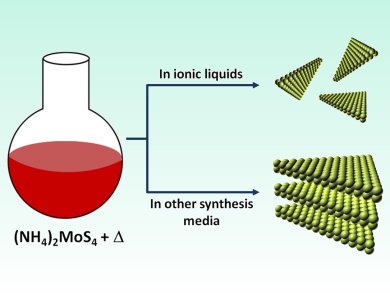
Ionic liquids are used far and wide, across catalysis and electrochemistry, waste recycling and fuels. Within catalysis alone, their uses span from coatings for porous glass catalysts to carbon dioxide fixation. A concept now emerging is the morphological control of crystal formation by ionic liquids. A recent report from Thomas Maschmeyer and co-workers, University of Sydney, Australia, on heterogeneous MoS2 catalysts prepared in ionic liquids investigates this very phenomenon.
MoS2 has also recently been proposed as an electrocatalyst for the hydrogen evolution reaction (HER) and could be a key player in catalyst design and the future “hydrogen economy”. This report describes how ionic liquids boost the activity of an MoS2 catalyst in the HER by inducing smaller particle sizes, delaminated morphology, and well-defined edges.
This system has overcome a common problem in catalysis: the balance between the active site population on the catalytic surface and instability owing to coordinative unsaturation. The crystallization of MoS2 in an ionic liquid as opposed to conventional solvents fosters increased active site stability and catalytic performance.
- Promoting the Formation of Active Sites with Ionic Liquids: A Case Study of MoS2 as Hydrogen-Evolution-Reaction Electrocatalyst,
V. Wing-hei Lau, A. F. Masters, A. M. Bond, T. Maschmeyer,
ChemCatChem 2011, 3, 1739–1742.
DOI: 10.1002/cctc.201100212