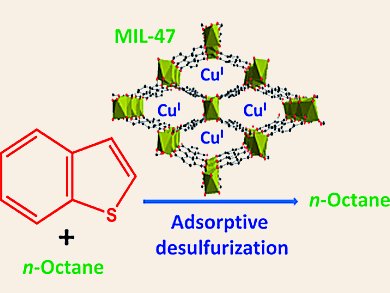
There is a considerable demand to reduce the content of sulfur-containing compounds such as thiophene (Th), benzothiophene (BT), and dimethyldibenzothiophene (DMDBT) in fuels like diesel and gasoline to a low level to prevent air pollution and deactivation of catalysts. Adsorption has been regarded as one of the most competitive methods. Little has been understood for the high uptake of S-compounds with modified metal-organic framework materials (MOFs) as adsorbents.
Nazmul Abedin Khan and Sung Hwa Jhung, Kyungpook National University, Daegu, Korea, have shown the remarkable adsorption capacity for BT over a modified MOF, CuCl2-loaded MIL-47 (MIL = materials of the Institute Lavoisier). MIL-47 is a typical MOF composed of vanadium and benzenedicarboxylate (BDC). The adsorption capacity of CuCl2(0.05)/MIL-47 is 122 % higher than that of CuI-Y zeolite that showed the highest capacity so far.
The modified MOF shows an unexpected reduction ability to form CuI ions from loaded CuII ions probably because of VIII in MIL-47. The obtained CuI ions show a beneficial effect on the adsorption of BT probably through π-complexation.
The adsorbent which can be readily prepared without calcination and reduction at high temperature, is promising for the adsorptive removal of harmful materials like S-compounds.
- Remarkable Adsorption Capacity of CuCl2-Loaded Porous Vanadium Benzenedicarboxylate for Benzothiophene,
Nazmul Abedin Khan, Sung Hwa Jhung,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201105113