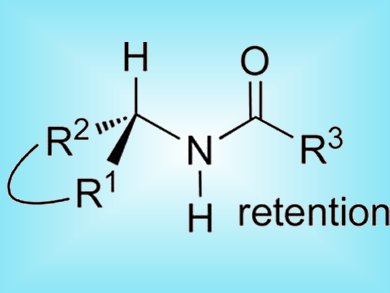
Interest in the direct transformation of chiral secondary alcohols into new compound classes with retention of configuration is growing as non-racemic alcohols are becoming increasingly available. Most stereoselective approaches to amides from chiral alcohols require multistep procedures.
Salvatore Lepore and colleagues, Florida Atlantic University, USA, report a one-pot amidation reaction for cyclic alcohols that gives complete retention of configuration. They use a chlorosulfite leaving group formed in situ by reaction of the alcohol and thionyl chloride. The leaving group is chelated by a TiIV nitrile complex that is also generated in situ by reaction of TiF4 and alkyl or aryl nitrile.

The Ti nitrile complex is thought to chelate the chlorosulfite in the transition state to create a carbocation that is rapidly captured by the nitrile nucleophile through a front-side attack mechanism. This is the first experimental verification of secondary hyperconjomers, a theory of non-planar carbocations developed by Sorensen and Schleyer.
- A Direct and Stereoretentive Synthesis of Amides from Cyclic Alcohols
D. Mondal, L. Bellucci, S. D. Lepore,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201101165