The chiral methyl carbinol moiety is present in a large number of natural products and biologically active compounds. Methyl Grignard reagents are anticipated to be highly reactive in the catalytic asymmetric addition of a methyl group to an aldehyde. Current techniques require more than stoichiometric amounts of chiral modifier, or an additional step in which the Grignard reagent is transmetalled into dimethylzinc or methyltitanium triisopropoxide.
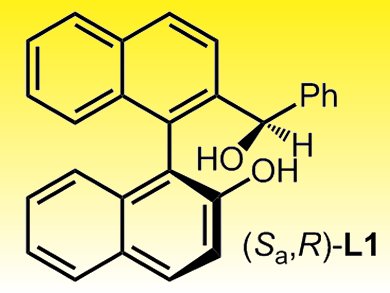
Miguel Yus and co-workers, Alicante University, Spain, report a facile one-pot methodology for the addition of MeMgBr to different aldehydes. They use a readily available chiral binaphthyl ligand in catalytic amounts and an excess of titanium tetraisopropoxide was found to be key.

This methodology provides the highest enantioselectivities and yields reported so far for this process, up to 99 % yield and 96 % ee. Moreover, Grignard reagents with longer alkyl chains could also be used in the enantioselective alkylation of a wide variety of aldehydes.
- Catalytic Enantioselective Addition of MeMgBr and Other Grignard Reagents to Aldehydes
E. Fernández-Mateos, B. Maciá, D. J. Ramón, M. Yus,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201101283