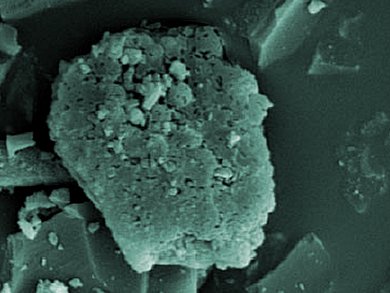
Chitosan is a biocompatible polysaccharide obtained from deacetylation of chitin. It can chemically or physically entrap various metal ions through its amine and hydroxyl groups. To use it for removal of metal ions in wastewater treatment, chitosan must be chemically modified with different crosslinking reagents, such as glutaraldehyde and epichlorohydrin. Such chemical modification reduces the adsorption capacity of chitosan.
Huacai Ge and Xiaohe Fan, South China University of Technology, Guangzhou, prepared a novel activated carbon-chitosan complex adsorbent (ACCA) via the crosslinking of glutaraldehyde and activated carbon-(NH2-protected) chitosan complex under microwave irradiation. The ACCA had higher adsorption capacity for Pb2+ and Cd2+ than chitosan due to larger porosity and surface area. The Pb2+ and Cd2+ ions could be desorbed efficiently, making ACCA recyclable.
These results have important implications for the design of low-cost and effective adsorbents in the removal of heavy metal ions from wastewaters.
- Adsorption of Pb2+ and Cd2+ onto a Novel Activated Carbon-Chitosan Complex,
H. Ge, X. Fan,
Chem. Eng. Technol. 2011, 34 (10), 1745–1752.
DOI: 10.1002/ceat.201000182