The catalytic properties of transition metal complexes depend on ligand parameters such as steric and electronic properties, bite angle, and chirality. Supramolecular chemistry provides new strategies for influencing ligand parameters and consequently the properties of their transition metal complexes.
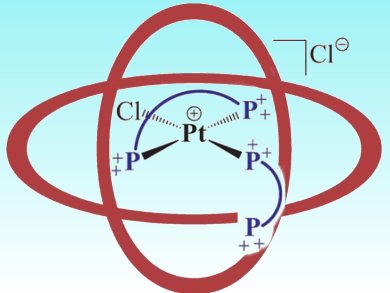
Joost Reek and colleagues, University of Amsterdam, The Netherlands, have demonstrated that supramolecular capsules formed from diphosphane ligands and calix[4]arenes influence the coordination geometry around a platinum atom. The geometry could be controlled through the amount of calix[4]arene present, with both the monoligated cis-Pt complex and the bisligated biscalix[4]arene trans-Pt capsule (pictured) being formed.
Metals encapsulated in this way are still available for catalytic transformations and this coordination environment — and the easy control over it — opens new opportunities to control the activity, stability and selectivity of potential homogeneous catalysts.
Image: © Wiley-VCH
- Control of the Coordination Geometry Around Platinum by a Supramolecular Capsule
T. S. Koblenz, H. L. Dekker, C. G. de Koster, P. W. N. M. van Leeuwen, J. N. H. Reek,
Eur. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201100809