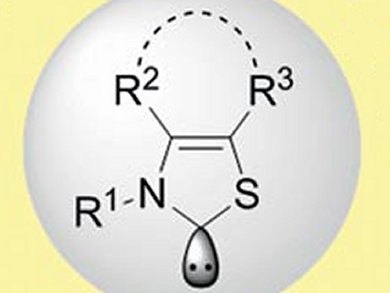
N-Heterocyclic carbene (NHC) organocatalysis has attracted considerable attention due to facile reaction handling, environmental friendliness, and low cost and toxicity compared with transition-metal catalysts. In each NHC-catalyzed transformation, the proper choice of NHC is crucial for success; the development of structurally related families of NHCs with slightly different properties is, therefore, of great interest.
Frank Glorius and colleagues, Westfälische Wilhelms-University, Münster, Germany, have synthesized a family of thiazolylidenes-based NHCs. The NHCs are based on a N-mesityl-substituted thiazolylidene with a cycloheptane backbone. The backbone architecture was varied by employing different arylamines and α-bromo ketones in a two-step synthesis. The NHCs are effective organocatalysts for promoting the chemoselective intermolecular cross-benzoin condensation leading to unsymmetrically substituted benzoins and may be applicable to other organocatalytic processes.
- A Family of Thiazolium Salt Derived N-Heterocyclic Carbenes (NHCs) for Organocatalysis: Synthesis, Investigation and Application in Cross-Benzoin Condensation
I. Piel, M. D. Pawelczyk, K. Hirano, R. Fröhlich, F. Glorius,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201100870