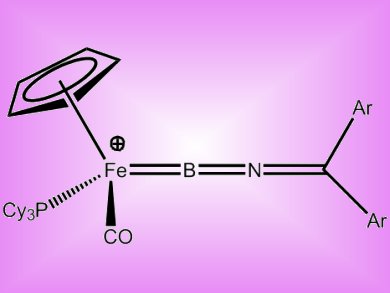
Boron donor ligands isoelectronic with classical organometallic systems are of interest in novel stoichiometric and catalytic reactions. The unusual ambiphilic character of the allenylidene ligand [LxM=C=C=CR2], which displays alternating electrophilic/nucleophilic reactivity at successive carbon centers, makes it interesting for a range of synthetically valuable organic transformations.
Simon Aldridge and co-workers, University of Oxford, UK, have reported the first iminoborylene complexes — isoelectric BN-containing analogues of allenylidene. The complexes, [CpFe(CO)(PCy3)(BNCAr2)]+, were synthesized by halide abstraction from 2 (see below). DFT calculations supported alternating α (boron),γ (carbon) electrophilicity similar to allenylidenes, however both boron complexes reacted with nucleophiles primarily at the sterically less hindered α (boron) center.
This unpredicted reactivity could lead to new routes to a range of organic compounds or their boron-containing analogues.

- Extending the Chain: Synthetic, Structural, and Reaction Chemistry of a BN Allenylidene Analogue
J. Niemeyer, D. A. Addy, I. Riddlestone, M. Kelly, A. L. Thompson, D. Vidovic, S. Aldridge,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201103757