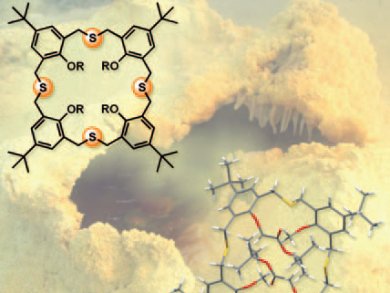
Homothiacalixarenes are similar to the calix[n]arene macrocycles commonly used in host–guest chemistry. They differ in that the calixarene’s methylene bridges are partly or completely replaced by CH2SCH2 bridges. Homothiacalixarenes have received little attention to date, despite the fact that their structural features are particularly attractive for supramolecular host–guest chemistry.
Wim Dehaen and co-workers, Katholieke Universiteit Leuven, Belgium, optimized the synthetic macrocyclization protocols to obtain homothiacalix[4]arene by a straightforward one-pot macrocyclization approach that involves substitution reactions of 1,3-bis(mercaptomethyl)benzene nucleophiles on 1,3-bis(bromomethyl)benzene derivatives under basic conditions. Homothiacalix[4]arenes were obtained with improved yields of up to 62 % compared with previous syntheses. This allowed their structures to be characterized by solution-phase NMR spectroscopy and solid-state X-ray spectroscopy.
This new protocol will hopefully lead to the increased use of homothiacalixarenes in host–guest chemistry such as in chemical sensors.
- Homothiacalix[4]arenes: Synthetic Exploration and Solid-State Structures
J. Thomas, K. Van Hecke, K. Robeyns, W. Van Rossom, M. P. Sonawane, L. Van Meevelt, M. Smet, W. Maes, W. Dehaen,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201101690