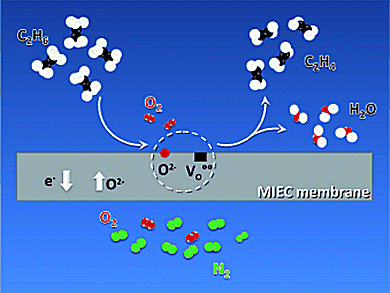
Ethylene is a key intermediate product in industrial chemistry. The current method for its production is steam cracking of ethane, naphtha, or heavier feedstocks, which is energy intensive, involves side reactions, and discontinuous operation is necessary for reactor cleanup. A potential alternative is the oxidative dehydrogenation of ethane (ODHE).
Jose M. Serra and colleagues, Universidad Politecnica de Valencia, Spain, achieved high ethylene productivity through the ODHE in a catalytic membrane reactor based on the highly solid-state oxygen permeable material Ba0.5Sr0.5Co0.8Fe0.2O3–δ. Ethylene is selectively produced:
- By preventing the direct contact of molecular oxygen and hydrocarbons, thereby minimizing the oxygen concentration in the reaction side,
- By properly selecting the temperature and inlet gas flow rates,
- By using methane as a diluting agent to achieve high ethylene yields.
The synergy between oxygen permeation, catalytic oxidation, and fluid dynamics at the membrane surface and reactor chamber is supposed to be responsible for the obtained exceptional results.
This membrane reactor system can be applied for other oxidative dehydrogenation reactions such as propane or butane dehydrogenation.
- High Ethylene Production through Oxidative Dehydrogenation of Ethane Membrane Reactors Based on Fast Oxygen-Ion Conductors,
M. P. Lobera, S. Escolastico, J. M. Serra,
ChemCatChem 2011.
DOI: 10.1002/cctc.201100055