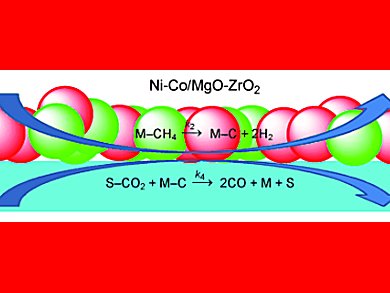
Dry reforming of methane using carbon dioxide utilizes carbon dioxide and methane whilst producing the valuable chemical precursor syngas. Carbon dioxide reforming, or dry reforming, produces syngas in a H2/CO ratio close to unity, which fulfills a requirement of the Fischer–Tropsch synthesis.
Nickel-based catalysts are known to be active in this reaction but have limited application due to severe catalyst deactivation. Recently, several researchers have reported the use of bimetallic catalysts in dry reforming, finding them to accumulate significantly reduced amounts of coke. Literature for other bimetallic catalysts is limited.
Subhash Bhatia and colleagues, Universiti Sains Malaysia, Pulau Pinang, tested a series of bimetallic catalysts containing nickel supported over MgO–ZrO2 for activity in the dry reforming of carbon dioxide. A nickel–cobalt bimetallic catalyst gave the best performance in terms of conversion and coke resistance from a range of Ni–X bimetallic catalysts, X = Ca, K, Ba, La, and Ce.
- Utilization of Greenhouse Gases through Dry Reforming: Screening of Nickel-Based Bimetallic Catalysts and Kinetic
Studies,
Mun-Sing Fan, Ahmad Zuhairi Abdullah, Subhash Bhatia,
ChemSusChem 2011.
DOI: 10.1002/cssc.201100113