Organohydrosilanes are key building blocks for the assembly of organosilicon compounds. However, strong basic Grignard or organolithium reagents are generally needed for the regio- and stereo-defined synthesis of such compounds, which limits the substrate scope.
Xing-Zhong Shu and colleagues, Lanzhou University, China, have developed a reductive C(sp2)–Si coupling approach to produce (hetero)aryl- and alkenyl hydrosilanes (pictured below). The team used nickel as a catalyst, 3,4,7,8-tetramethyl-1,10-phenanthroline or 2,2′-bipyridine as a ligand, manganese as a reducing agent, and dimethylacetamide as a solvent. This reaction between chlorohydrosilanes and carbon electrophiles involves an Si–Cl cleavage—in contrast to “conventional” organochlorosilane transformations, where transition metals activate the Si–H bond.

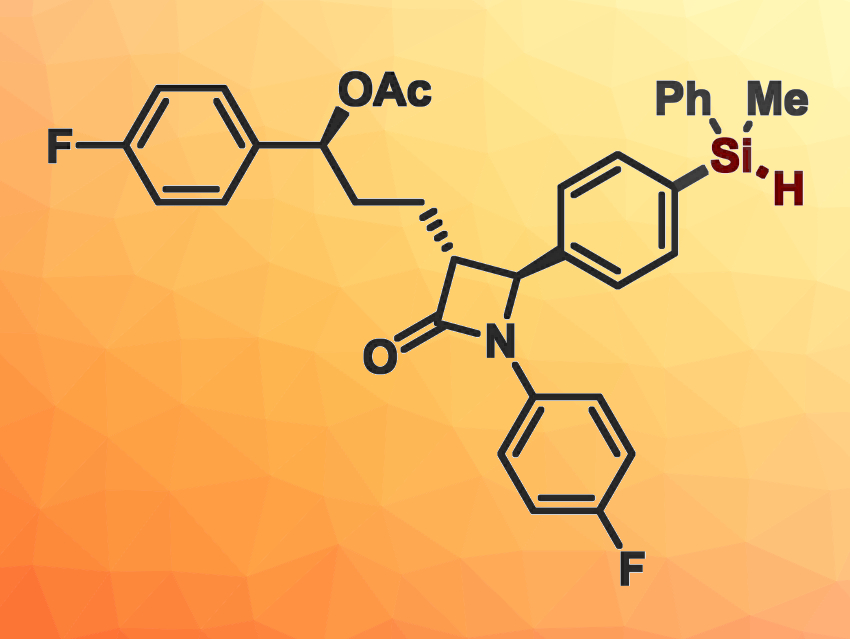
The reaction provides access to various functionalized (hetero)aryl- and alkenylhydrosilanes, many of which are difficult to produce otherwise, under mild conditions. This method offers solutions to several challenges in organohydrosilane synthesis, including the incorporation of –SiHR2 units into complex, biologically active molecules (example pictured at the top).
- Nickel‐Catalyzed Reductive C(sp2)−Si Coupling of Chlorohydrosilanes via Si−Cl Cleavage,
Zhen‐Zhen Zhao, Xiaobo Pang, Xiao‐Xue Wei, Xue‐Yuan Liu, Xing‐Zhong Shu,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202200215