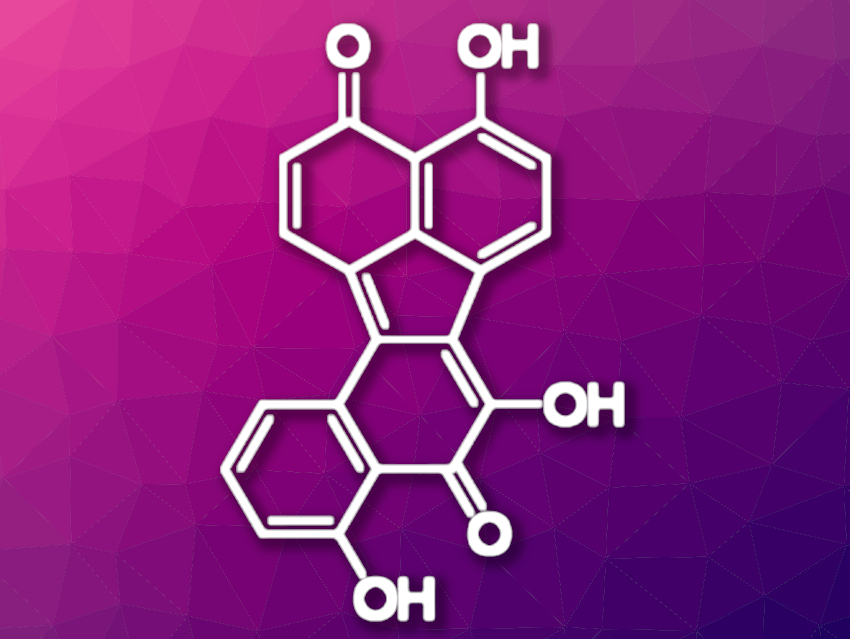
Bulgarein (pictured above) is a bioactive blue pigment with a benzo[j]fluoranthene skeleton that was first isolated from the fungus Bulgaria inquinans. Benzo[j]fluoranthene itself is a well-known toxic polycyclic aromatic hydrocarbon (PAH) that is present in fossil fuels and also released during the incomplete combustion of organic matter. Several other compounds related to bulgarein are known. Nevertheless, so far only a few papers have been published about the synthesis of compounds with a benzo[j]fluoranthene skeleton.
Patrick Swieca and Peter Spiteller, University of Bremen, Germany, have developed an efficient and short total synthesis of bulgarein (pictured below). Two known naphthalene derivatives were coupled in the first step via a Suzuki–Miyaura reaction to give the corresponding dinaphthalene derivative. This intermediate was then converted to the desired benzo[j]fluoranthene skeleton via an acid-induced ring-closing reaction.

An oxidation of the resulting molecule and a final deprotection step gave bulgarein in only for steps with an overall yield of 25 %. The developed total synthesis makes bulgarein available on a 100-milligram scale, which should be sufficient for further bioactivity studies.
- Total Synthesis of Bulgarein,
Patrick Swieca, Peter Spiteller,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202101576



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)