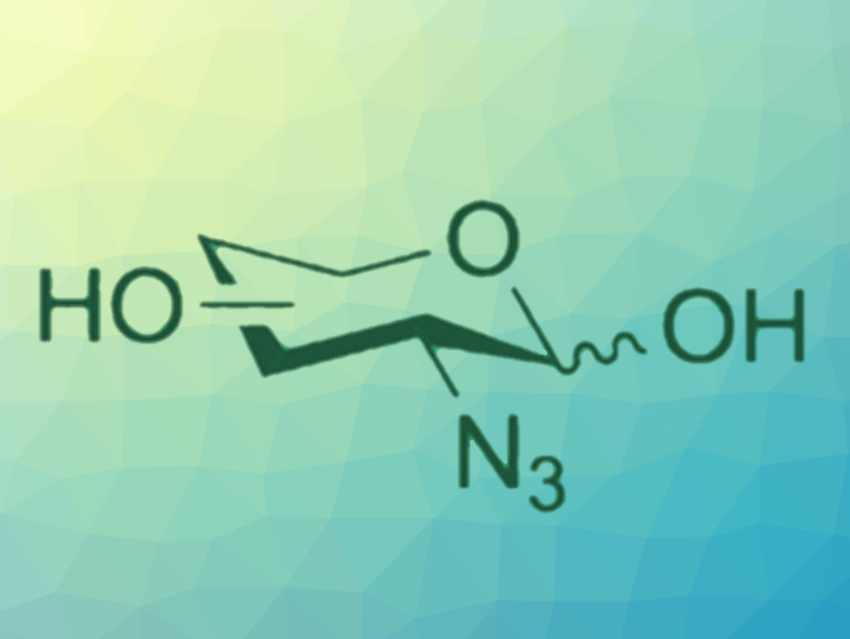
Azide-containing sugars are important building blocks in carbohydrate chemistry and can be useful probes in chemical biology. Traditional strategies to install azide groups in sugars generally use harsh and/or hazardous conditions and often require long reaction times.
Chantelle Capicciotti, Queen’s University, Kingston, Canada, and colleagues have developed a high-yielding diazotransfer protocol that transforms amine-containing sugars into the corresponding azido-sugars (pictured below) in under five minutes and up to quantitative yields. This reaction uses fluorosulfuryl azide (FSO2N3) as a diazotransfer reagent together with CuSO4 as a catalyst. The copper ions also act as a color indicator to monitor the reaction progress. The color of the solution changes from blue to green when the reaction is complete and the amine is completely consumed.

The team found that this diazotransfer is compatible with various amine-containing carbohydrates. The method avoids many of the safety concerns associated with traditional azido-nitration and diazotransfer strategies. This efficient route to azide-containing sugars could expand access to this functional group for a wide range of applications.
- Efficient Synthesis of Azido Sugars Using Fluorosulfuryl Azide Diazotransfer Reagent,
Joshua Kofsky, Gour Daskhan, Matthew Macauley, Chantelle Capicciotti,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202200108