Chiral homoallylic alcohols are valuable building blocks in synthetic chemistry. They can be prepared, e.g., via the nucleophilic allylation of aldehydes. There are enantioselective variants of this reaction, but an asymmetric allylation of aldehydes with stannanes using a Pd(II) catalyst had not yet been reported. In addition, existing methods for enantioselective Lewis-acid-catalyzed allylations can have issues such as a need for high catalyst loadings and long reaction times.
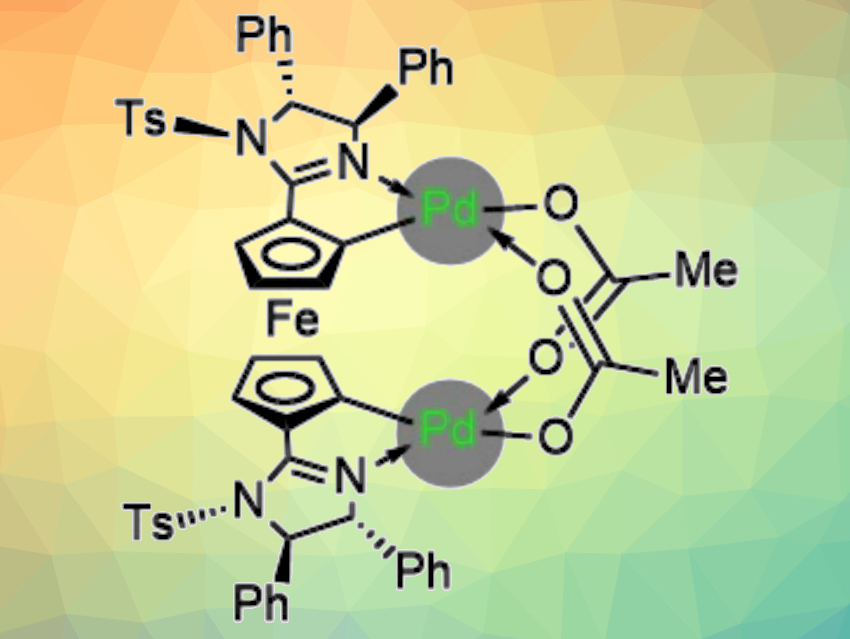
René Peters, University of Stuttgart, Germany, and colleagues have used chiral ferrocene bisimidazoline bispalladacycle complexes (pictured) as the first palladium catalysts capable of controlling asymmetric nucleophilic allylations of aldehydes with allyltributyltin. Using these catalysts, the team observed unprecedented turnover numbers (TONs) up to 620. The desired alcohols were obtained in generally high yields and with high enantioselectivities.
According to the researchers, the catalyst likely undergoes a transmetallation with the allyl stannane to form a nucleophilic η1-allyl palladium species. A large variety of substrates is tolerated, and even challenging electron-rich aldehydes can be converted into the corresponding homoallylic alcohols. Thus, the method could provide a valuable tool to overcome synthetic challenges.
- Bispalladacycle Catalyzed Nucleophilic Enantioselective Allylation of Aldehydes by Allylstannanes,
Martin Heberle, Sarah Legendre, Nick Wannenmacher, Manuel Weber, Wolfgang Frey, René Peters,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200093