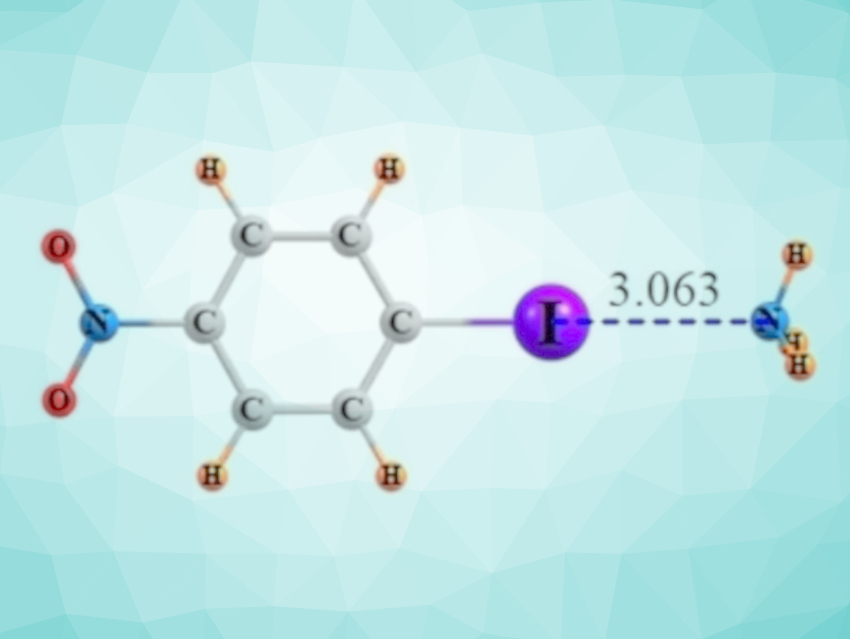
Halogen bonds are similar to the well-known hydrogen bonds and can have comparable strengths. These interactions are due to a small positively charged region that develops on an otherwise negatively charged halogen atom. This positively charged area can then form an attractive interaction with a nucleophile. The large iodine atom engages in the strongest halogen bonds, and it commonly does so when attached to an aromatic ring.
Steve Scheiner and Sarah Hunter, Utah State University, Logan, USA, have used quantum chemical calculations to investigate the effects of substituents in iodobenzene derivatives on the strength of the halogen bond formed by the I atom with a base, in this case, NH3 (example pictured). The substituents N(CH3)2, NH2, CH3, OCH3, COCH3, Cl, F, COH, CN, and NO2 were each placed in ortho-, meta-, and para position relative to the I atom in the calculations.
The team found that the halogen bond is weakened by electron-donating groups like NH2 or CH3, while electron-withdrawing agents such as CN and NO2 strengthen the interaction. These substituent effects are most pronounced when the substituents are in ortho position to the I atom, followed by the meta– and para positions. The researchers observed that NMR measurements can provide an experimental gauge for the strength of the halogen bond: The chemical shift of the C and N atoms next to the iodine and the coupling constant between I and N are correlated most tightly with the bond strength.
- Influence of Substituents in the Benzene Ring on the Halogen Bond of Iodobenzene with Ammonia,
Steve Scheiner, Sarah Hunter,
ChemPhysChem 2022.
https://doi.org/10.1002/cphc.202200011