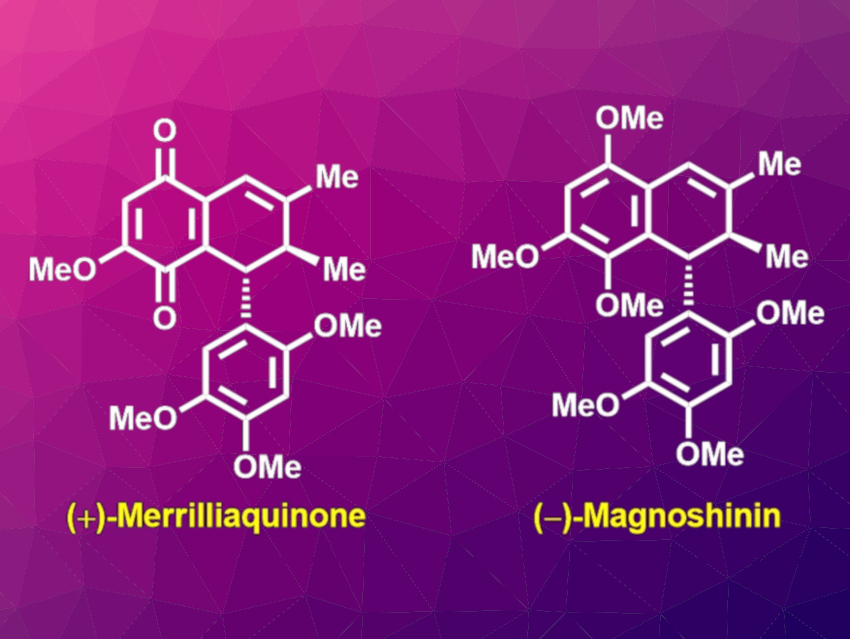
Merrilliaquinone (pictured left) is a rare natural product with a dihydronaphthoquinone scaffold. It was isolated in racemic form from the plant Illicium merrillianum. The selective cytotoxicity of (+)-merrilliaquinone towards human hepatoma cells over the normal liver cells makes it a potential lead for anti-cancer drug development.
Magnoshinin (pictured right) is a natural product with a dihydronaphthalene scaffold and shows anti-inflammatory activity. It was isolated from the plant Magnolia salicifolia. Magnoshinin could be a possible biosynthetic precursor for merrilliaquinone.
On the basis of this hypothesis, Tabrez Khan, Indian Institution of Technology Bhubaneswar, Khurdha, India, and colleagues have developed a unified approach for the first asymmetric total syntheses of (−)-magnoshinin and (+)-merrilliaquinone. The team first prepared the chiral tetralin core, starting from 3-(2,4,5-trimethoxyphenyl)propanoic acid and using a relayed asymmetric induction strategy.
The researchers then used a chemoselective, late-stage oxidative functionalization protocol employing 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to obtain (−)-magnoshinin, which can be converted to (+)-merrilliaquinone via an oxidative demethylation. These two products were obtained in overall yields of 28.2 % and 20.6 %, respectively.
- Total Synthesis of (−)‐Magnoshinin and (+)‐Merrilliaquinone: Application of a Late‐Stage Oxidative Functionalization Protocol,
Abdus Salam, Dileep Kumar, Tonish K. Sahu, Rahimuddin Khan, Tabrez Khan,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202101452