Julius F. Kögel, Universität Bremen, Germany, and colleagues have reacted the bulky perfluorinated trityl alcohol (C6F5)3COH with different metal precursors, i.e., Na(N(SiMe3)2), Mg(nBu2), ZnEt2, or Ti(OiPr)4. The team also reacted the lithium salt (C6F5)3COLi with AgBF4 or TlF in CH2Cl2 at room temprature.
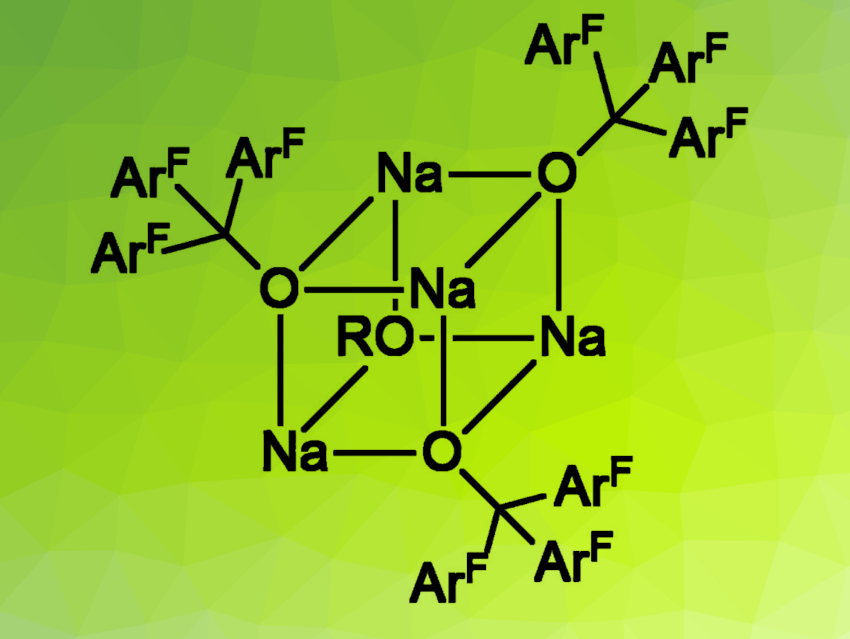
The team used single-crystal X-ray diffraction to characterize the products (example pictured above). They found a rich variety of different shapes for the synthesized metal complexes, which all contain the anionic ligand [(C6F5)3CO]–, e.g., a cube, a tetrahedron, a square, a rhombus, and a butterfly structure (simplified structures pictured below).

The discovered structures show interesting properties, such as interactions between the metal centers and carbon-bound fluorine atoms. According to the researchers, this work improves the understanding of the structural chemistry of [(C6F5)3CO]– and could provide novel precursors for the preparation of new Lewis superacids.
- Metal Complexes of the Perfluorinated Trityl Alkoxide [(C6F5)3CO]−,
Julius F. Kögel, Felix Feige, Daniel Duvinage, Enno Lork, Alexey Y. Timoshkin, Jens Beckmann,
Eur. J. Inorg. Chem. 2022.
https://doi.org/10.1002/ejic.202100872