Phosphorus compounds have applications, e.g., in the pharmaceutical industry. They are also widely used as ligands in catalysis. Therefore, it is important to investigate and understand reaction mechanisms of phosphorus derivatives. Phosphoranides, for example, are interesting hypervalent species that could serve as model compounds for intermediates or transition states in nucleophilic substitution reactions at trivalent phosphorus substrates.
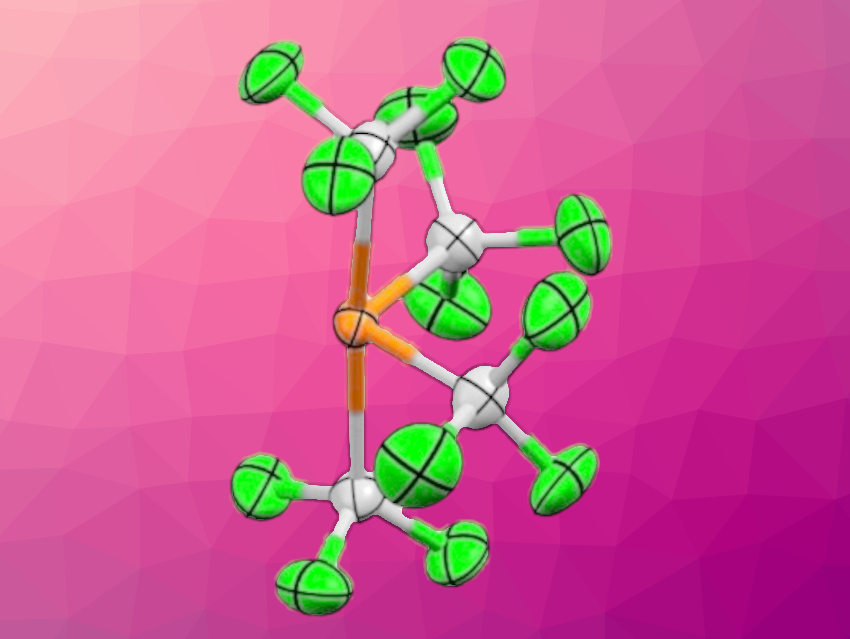
Gerd-Volker Roeschenthaler, Jacobs University Bremen, Germany, and colleagues have prepared stable trifluoromethylphosphoranide salts. The team reacted trivalent precursors, i.e., P(CF3)3 or PF2(CF3), with sources of F– or CF3– such as Me3SiCF3, KF, or NMe4F. P(CF3) was reacted in the presence of 18-crown-6. The researchers obtained [K(18-crown-6)][P(CF3)4] (anion pictured), [K(18-crown-6)][P(CF3)3F], and [NMe4][P(CF3)2F2].
The obtained phosphoranides can be considered models that represent transition states of nucleophilic attacks on trivalent phosphorus centers. Fluorinated substituents increase the stability of the anions, however, the phosphoranides are still sensitive towards temperature, moisture, and oxygen. The compounds with crown-ether-complexed potassium ions could be characterized using single-crystal X-ray analysis. The solid-state structures of trifluoromethyl phosphoranides are based on distorted trigonal bipyramids, with the lone pair at the phosphorus center occupying one of the equatorial positions.
- Synthesis, Reactivity and Structural Properties of Trifluoromethylphosphoranides,
Oleg O. Shyshkov, Alexander A. Kolomeitsev, Berthold Hoge, Enno Lork, Axel Haupt, Mira Keßler, Gerd-Volker Roeschenthaler,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202104308