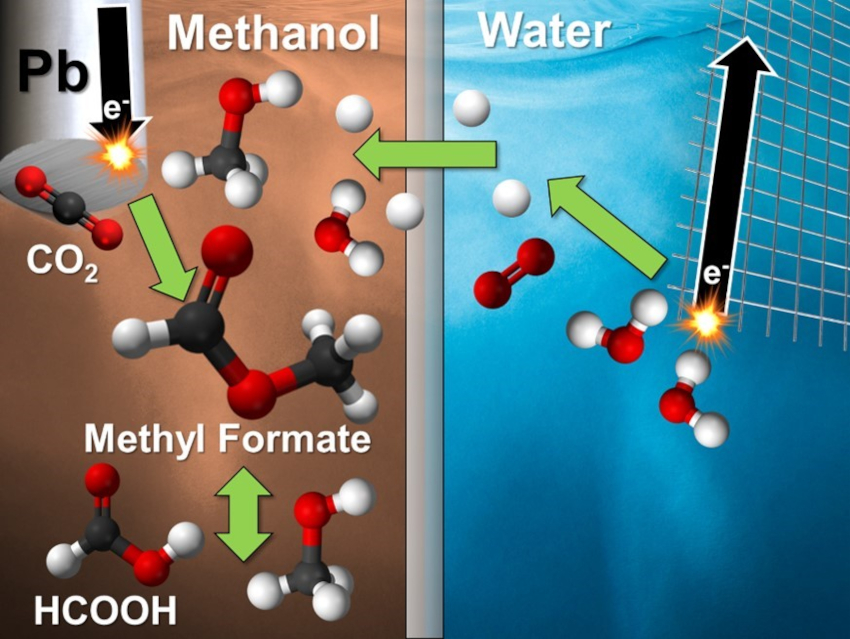
The electroreduction of CO2 is an attractive way to decrease greenhouse gas emissions while producing valuable chemicals. Methanol is an often-overlooked solvent for electrochemical CO2 reduction despite its increased CO2 solubility compared to water, water-like reactivity, and ability to form a unique and valuable product: methyl formate.
Craig A. Grapperhaus, Joshua M. Spurgeon, University of Louisville, KY, USA, and colleagues have developed an electrochemical cell that can produce methyl formate with high selectivity via the reduction of CO2 in methanol (pictured). The team used a Pb wire cathode in KCl-saturated methanol and observed high selectivity for CO2 reduction products (formate, formic acid, and methyl formate) under different electrolyte conditions. Formate is predominantly formed at high pH (>5), formic acid at intermediate pH (3–5), and methyl formate formation occurs at low pH (<3).
According to the researchers, Pb is effective for this process because it can catalyze the reduction reaction while largely suppressing hydrogen production, which is the major side reaction that typically dominates in acidic media. The work could be a first step towards an efficient route to methyl formate via the electroreduction of CO2.
- The pH and Potential Dependence of Pb‐Catalyzed Electrochemical CO2 Reduction to Methyl Formate in a Dual Methanol/Water Electrolyte,
Dillon T. Hofsommer, Ying Liang, Sandesh S. Uttarwar, Manu Gautam, Sahar Pishgar, Saumya Gulati, Craig A. Grapperhaus, Joshua M. Spurgeon,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202102289