The selective activation of inert C–C bonds is a critical step in deconstructing plastic waste. However, C–C bond activation under mild reaction conditions is difficult due to thermodynamic and kinetic constraints.
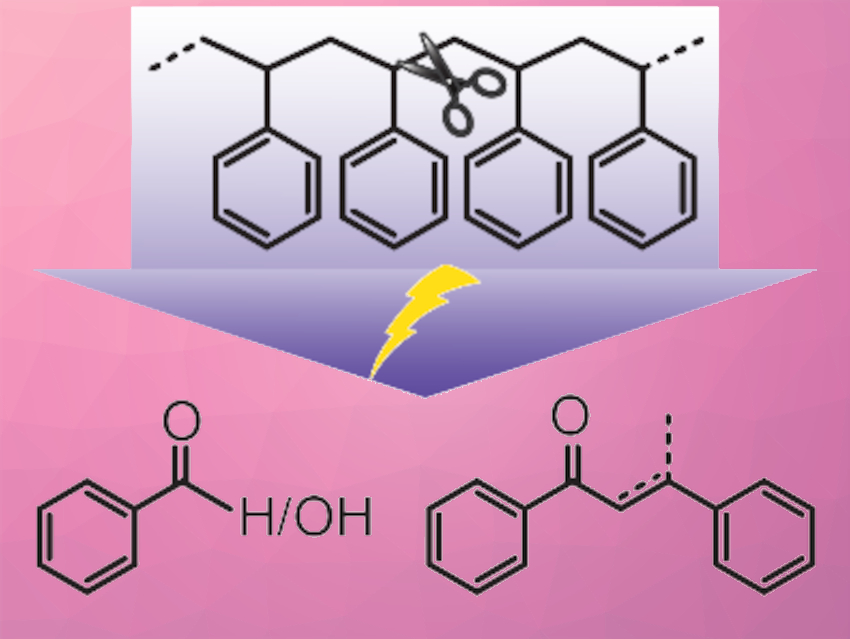
Yuriy Román, Massachusetts Institute of Technology (MIT), Cambridge, USA, and colleagues have used an electrochemical strategy to activate the inert C–C bonds in polystyrene (PS) at room temperature and ambient pressure. The team used N-hydroxyphthalimide (NHPI) as a redox mediator, which is oxidized to a phthalimide-N-oxyl (PINO) radical and then initiates hydrogen atom transfer (HAT) reactions with benzylic C–H bonds. The resulting carbon radical is captured by O2 to form a peroxide, which decomposes into oxygenated C–C bond-cleaved products (examples pictured). The PINO radical is oxidatively regenerated at the electrode.
This indirect approach to activating C–C bonds reduces the oxidation potential by >1.2 V compared to the direct oxidation of the substrate. Using this strategy, oligomeric styrene and PS were converted into oxygenated monomers, dimers, and oligomers. This work demonstrates the feasibility of electrochemical approaches for breaking inert chemical bonds under mild conditions. The results could provide a basis for plastic waste deconstruction strategies using renewable electricity.
- Electrochemical Activation of C–C Bonds via Mediated Hydrogen Atom Transfer Reactions,
Bing Yan, Changxia Shi, Gregg T. Beckham, Eugene Y.-X. Chen, Yuriy Román,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202102317



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)