Achieving mono-selectivity when there are two or more equivalent functional groups present can be a problem: Applying only one equivalent of the reagent generally fails due to an unsatisfactory statistical product distribution. An important example of such a reaction is the mono-hydrolysis of dicarboxylic esters. Using solubility effects or enzymatic transformations are established methods to improve selectivity.
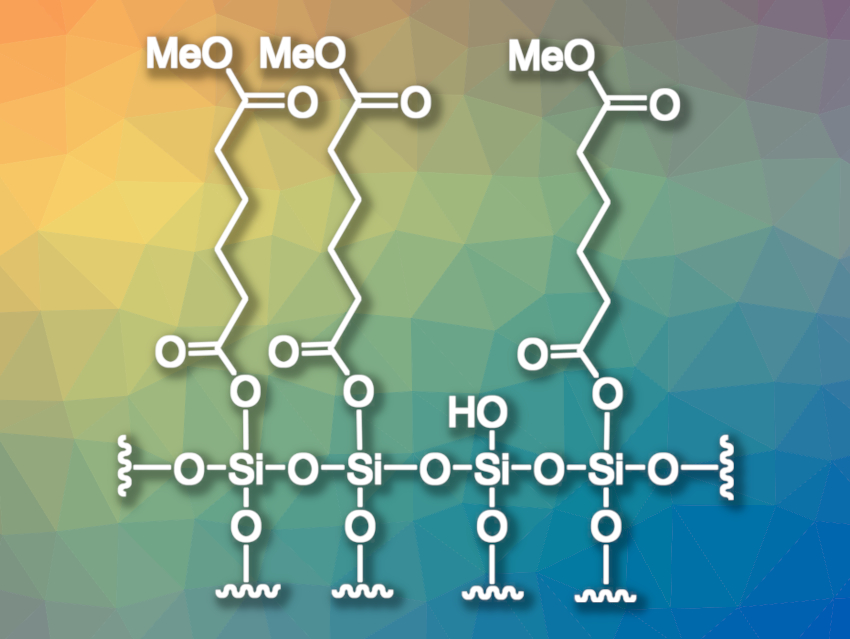
Gerald Dyker, Bochum University, Germany, has developed a method for the monohydrolysis of dicarboxylic esters. He tested the general idea of using temporary covalent bonds to a solid surface to achieve mono-selectivity. As a proof-of-concept, Dyker performed a thermolysis of dicarboxylic esters on the surface of silica gel, leading to surface-bound silyl ester units (pictured). Cleaving the bonds to the silica gel with acetone/methanol at reflux temperature then gave the desired monoesters in yields of 70–97 %.
The process can be used on a multigram scale with a minimalistic apparative setup. Even with flexible aliphatic substrates such as dodecanedioic acid dimethyl ester, the second ester functionality does not bind well to the surface, thus, the second hydrolysis is still inhibited. According to Dyker, the selectivity effects of temporary covalent bonds at the silica gel surface and other chemically modified solid surfaces are interesting for future work.
- Silica‐Mediated Monohydrolysis of Dicarboxylic Esters,
Gerald Dyker,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202101443