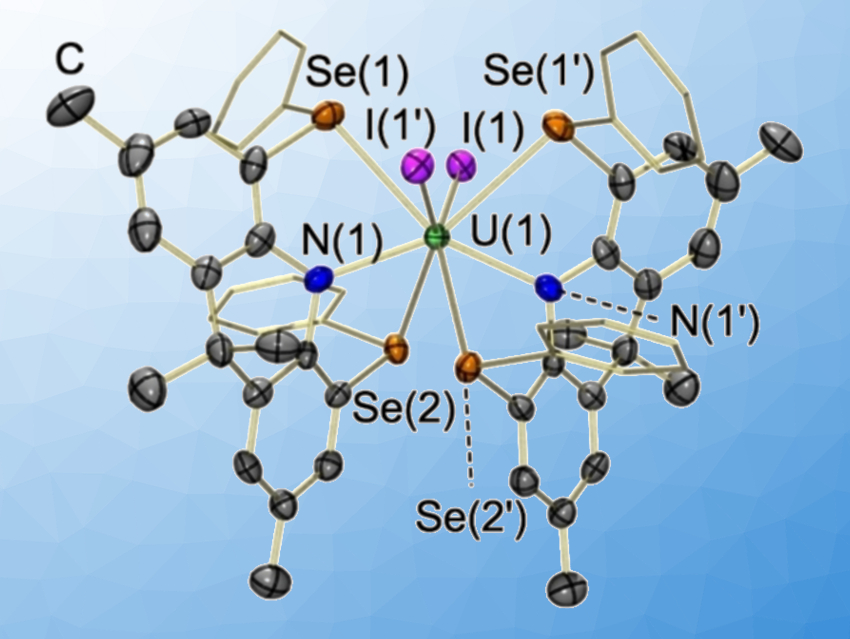
Understanding actinide–ligand bonding is important from a fundamental perspective, but also for the rational development of ligands for lanthanide-actinide separation in nuclear-fuel reprocessing. Uranium–sulfur interactions are typically more covalent than uranium–oxygen interactions. Thus, uranium–selenium interactions may be anticipated to be even more covalent. However, there are only a few examples of uranium–thioether (SR2) complexes, and uranium –selenoether (SeR2) complexes had not definitively been identified so far.
David J. H. Emslie, McMaster University, Hamilton, Ontario, Canada, and colleagues have synthesized the uranium complexes [(κ3-AS2Ph2)2UI2] and [(κ3-ASe2Ph2)2UI2], with H[AS2Ph2] = 4,5-bis(phenylsulfido)-2,7,9,9-tetramethylacridan and H[ASe2Ph2] = 4,5-bis(phenylselenido)-2,7,9,9-tetramethylacridan. The Se-containing complex (pictured) is the first example of a uranium–selenoether complex.
The team first prepared the pincer proligands H[AS2Ph2] and H[ASe2Ph2], which were deprotonated to obtain the potassium salts [K(AS2Ph2)(dme)] and [K(ASe2Ph2)(dme)2] (dme = 1,2-dimethoxyethane). Reactions of two equivalents of one of these salts with [UI4(dioxane)2] then gave the desired products. Computational analyses of the resulting complexes, as well as a hypothetical ether analogue, indicate an increase of covalent character in the U–ER2 bonds from O to S to Se.
- Uranium(IV) Thio‐ and Selenoether Complexes: Syntheses, Structures, and Computational Investigation of U−ER2 Interactions,
Novan A. G. Gray, Jeffrey S. Price, David J. H. Emslie,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103580