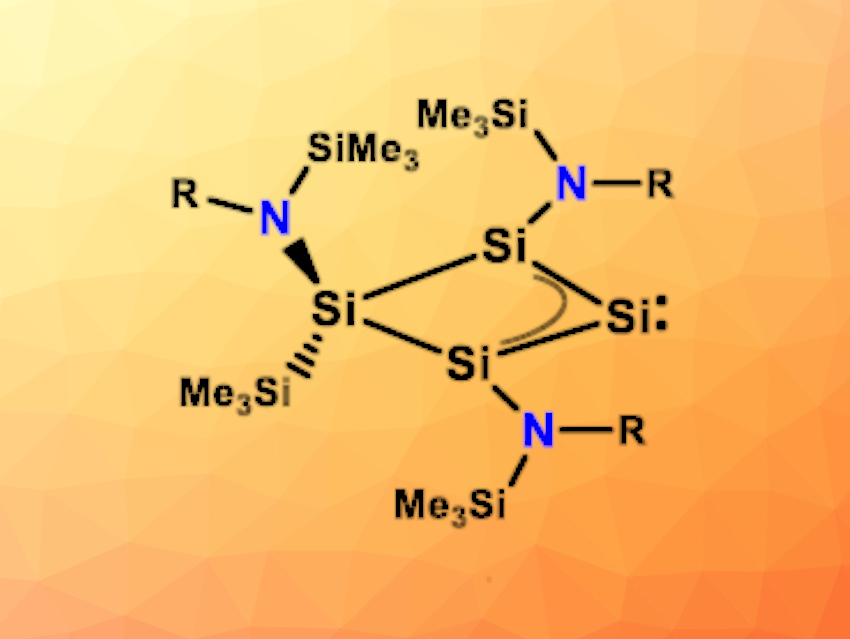
Silicon analogues of carbenes, i.e. silylenes, are interesting reactive species, e.g., for small-molecule activation. There are examples of acyclic and heterocyclic silylenes. However, an isolated neutral homocyclic silylene, where only other silicon atoms are connected to a twofold-coordinated silicon atom, had not been reported to date.
Felicitas Lips and colleagues, University of Münster, Germany, have found that the preparation of a homocyclic silylene (pictured below) can be achieved, starting from a four-membered bicyclic amido-substituted silicon(I) amide. First, a reduction with KC8 in the presence of [18]crown-6 cleaves an amido substituent. Then, the generated homoaromatic anion reacts with SiMe3Cl to give a homocyclic silylene.

The silylene possesses an electron-rich, nucleophilic character. According to density functional theory (DFT) calculations, the isolated homocyclic silylene is in equilibrium with a more reactive isomer via a skeletal rearrangement. This rearranged silylene reacts with methanol, ethene, phenyl- and diphenylacetylenes, as well as 2,3-dimethyl-1,3-butadiene. The researchers believe that an even wider range of reactions may be accessible with this new compound.
- Synthesis and Reactivity of a Neutral Homocyclic Silylene,
Felicitas Lips, Jan Keuter, Alexander Hepp, Anja Massolle, Johannes Neugebauer, Christian Mück-Lichtenfeld,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202114485