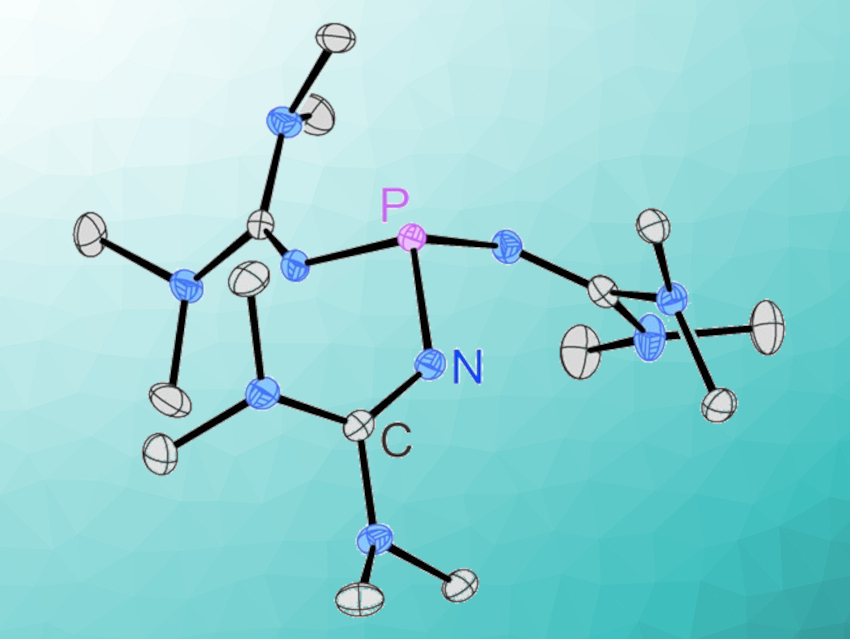
Phosphines are widely used in many areas of academic research, as well as in industrial applications. Superbasic phosphine derivatives have also garnered interest, e.g., in the fields of small molecule activation and catalysis. Arguably the simplest phosphorus superbase, tris(1,1,3,3-tetramethylguanidinyl)phosphine, however, has been elusive.
Fabian Dielmann, University of Innsbruck, Austria, and colleagues have developed a simple and scalable synthesis for this phosphine, starting from cheap and commercially available bulk chemicals (pictured below). The phosphine is obtained as a crystalline solid in 92 % yield by reacting PCl3 and P(NMe2)3 with 1,1,3,3-tetramethylguanidine, followed by the deprotonation of the resulting phosphonium salt with potassium bis(trimethylsilyl)amide (KHMDS).

The new phosphine is exceptionally basic (pKBH+(MeCN) = 32.7) and strongly electron-donating (Tolman electronic parameter (TEP) = 2049 cm–1). It forms reversible Lewis-base adducts with, e.g., carbon dioxide and sulfur dioxide, and coordinates to AuI, PdII, and RhI centers. Given the ease of the synthesis, the new phosphine has the potential to be useful in stoichiometric reactions requiring extremely basic phosphorus(III) species.
- Tris(tetramethylguanidinyl)phosphine: The simplest non‐ionic phosphorus superbase and strongly‐donating phosphine ligand,
Florenz Buß, Maike B. Röthel, Janina A. Werra, Philipp Rotering, Lukas F. B. Wilm, Constantin G. Daniliuc, Pawel Löwe, Fabian Dielmann,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202104021



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)