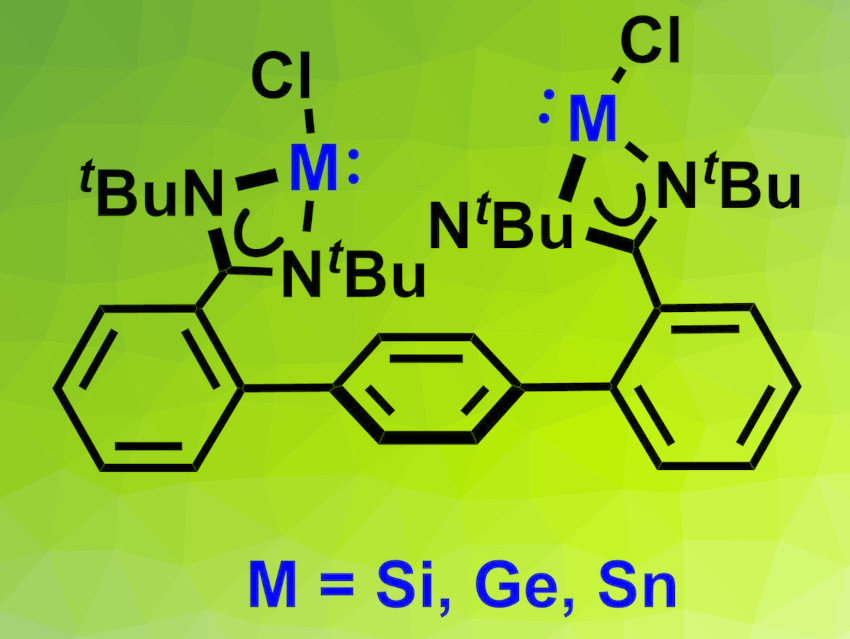
Bis(chlorotetrylene)s contain two low-valent main-group element centers. They can be considered heavier bis(carbene) analogues. As such, they can serve as chelating ligands and might be useful in the design of catalysts. The bridging of the two tetrel (Si, Ge, Sn) sites can be achieved in three different ways: via direct attachment of the linker to the tetrel(II) center, with ditopic N,N-chelating ligands bridged at nitrogen atoms, or with ditopic N,N-chelating ligands bridged at the backbone of the ligand. Examples of the latter two modes have been limited to Ge and Sn so far.
Robert Kretschmer, Friedrich Schiller University Jena, Germany, and colleagues have synthesized bis(chlorotetrylene)s of silicon, germanium, and tin that are bridged via the ligand’s backbone (pictured). The team started from 1,4-bis(2-bromophenyl)benzene, which was reacted with n-butyllithium and then with N,N‘-di-tert-butylcarbodiimide to obtain a suitable ligand precursor. This precursor was first converted to a bis(dichorosilane) intermediate and then to the desired bis(chlorosilylene). The germanium and tin compounds were prepared via a double deprotonation of the ligand precursor and a subsequent reaction with either GeCl2⋅dioxane or SnCl2.
Preliminary reactivity studies point to an individual rather than a cooperative activation by the two chlorosilylene centers. According to the researchers, they will further investigate to identify potential cooperative effects and study the use of all three products as chelate ligands towards main-group elements and transition metals.
- Dinuclear Chlorotetrylenes of Silicon, Germanium, and Tin Based on a Backbone‐Bridged Bis(amidine),
Maximilian Dehmel, Marius A. Wünsche, Helmar Görls, Robert Kretschmer,
Eur. J. Inorg. Chem. 2021.
https://doi.org/10.1002/ejic.202100692