The industrial synthesis of nitrogen-containing chemicals starts with the reduction of N2 to ammonia in the Haber–Bosch process. This reaction is energy-demanding and has a large carbon footprint. Direct oxidative pathways that avoid an initial reduction are desirable for high-valent products such as nitrogen oxides (NOx).
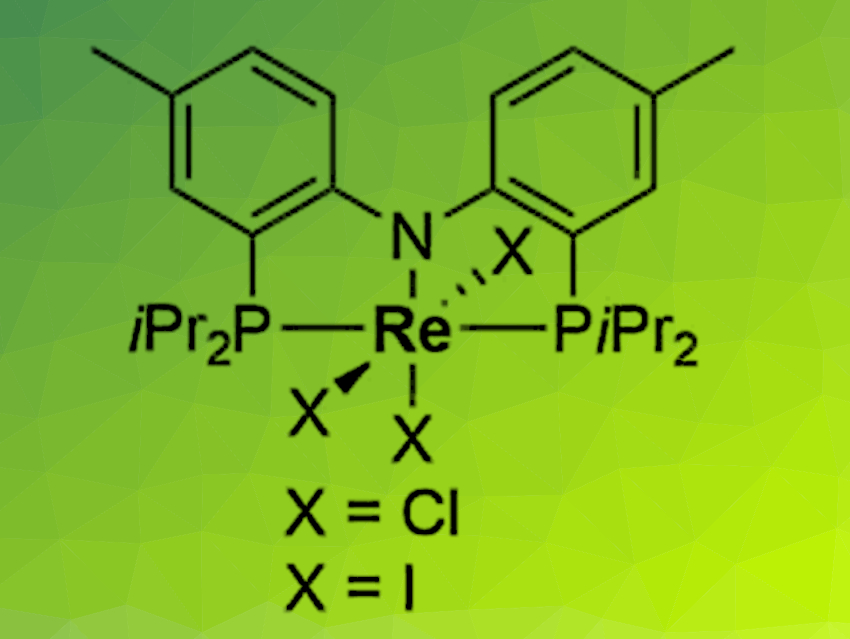
Sven Schneider, University of Göttingen, Max C. Holthausen, University of Frankfurt am Main, both Germany, and colleagues have developed a new approach for the synthesis of nitrous oxide (N2O) from N2 and nitric oxide (NO). The reaction is enabled by an electrochemically driven splitting of the extremely strong bond of N2 at a rhenium pincer complex (pictured). The resulting nitride reacts with NO to release N2O in nearly quantitative yield.

Quantum chemical calculations indicate that the N–NO coupling benefits from the initial coordination of NO at the metal, which activates the nitride group towards radical reactivity with additional NO. The reaction of N2 and NO via N2 splitting could provide a new approach towards the direct oxidative functionalization of N2 beyond the Haber–Bosch process.
- Rhenium‐Mediated Conversion of Dinitrogen and Nitric Oxide to Nitrous Oxide,
Lukas Alig, Kim A. Eisenlohr, Yaroslava Zelenkova, Sven Rosendahl, Regine Herbst‐Irmer, Serhiy Demeshko, Max C. Holthausen, Sven Schneider,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202113340