Helically fused aromatic compounds such as helicenes are useful to construct nonplanar chiral structures. The structures and properties of such helical compounds are considerably influenced by the kind of aromatic units incorporated in the framework.
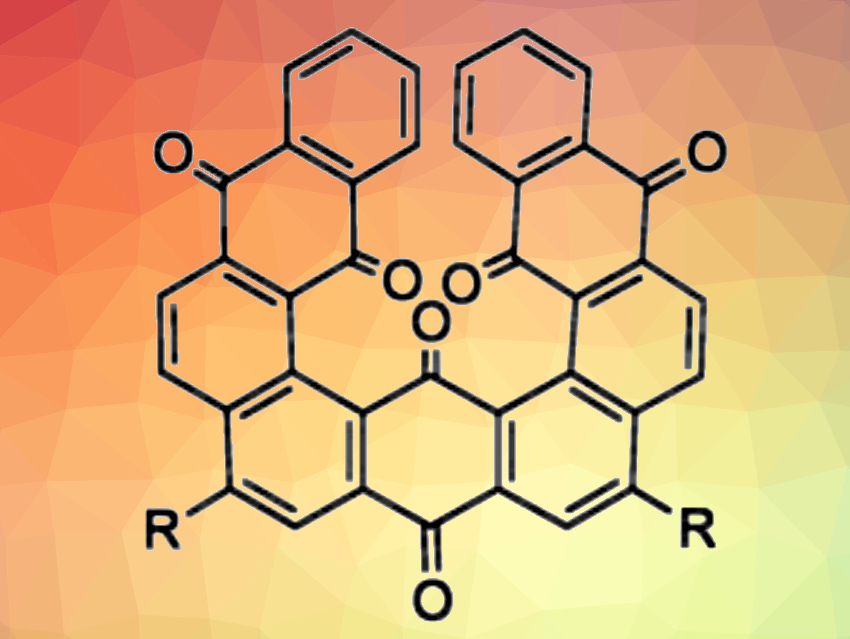
Shinji Toyota, Tokyo Institute of Technology, Japan, and colleagues have looked at what happens when anthraquinone, a typical aromatic ketone, is adopted as the building unit for helical structures. The team synthesized fused compounds consisting of three anthraquinone units (example pictured) by CrO3 oxidation of the corresponding fused anthracene compounds. The fused anthracene precursors were prepared using PtCl2-catalyzed cycloisomerizations. The researchers prepared the parent compound, as well as analogues with two 2,4,6-trimethylphenyl groups or two 4-(octyloxy)phenyl groups, respectively.
The products were characterized using single-crystal X-ray analysis, dynamic NMR measurements, and density functional theory (DFT) calculations. The team found that the products have helical structures with significant deformations of the aromatic frameworks and unusually close contacts between the inner carbonyl groups. The intermolecular C=O···C=O distance of 2.47 Å is one of the shortest in sterically congested diketones. The barrier to helical inversion is estimated to be 77 kJ mol–1. According to the researchers, this barrier is enhanced by the carbonyl groups.
- Synthesis, structures, and properties of helically fused anthraquinones with unusually close carbonyl–carbonyl contacts,
Kozue Morioka, Kan Wakamatsu, Eiji Tsurumaki, Shinji Toyota,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103694