Transition-metal-catalyzed remote functionalization can be achieved via “chain walking”—a synthetic technique that can be used to functionalize unreactive C–H bonds on a hydrocarbon chain. This method can be used to access complex building blocks that cannot be synthesized by traditional techniques. However, these processes generally require long reaction times and elevated temperatures.
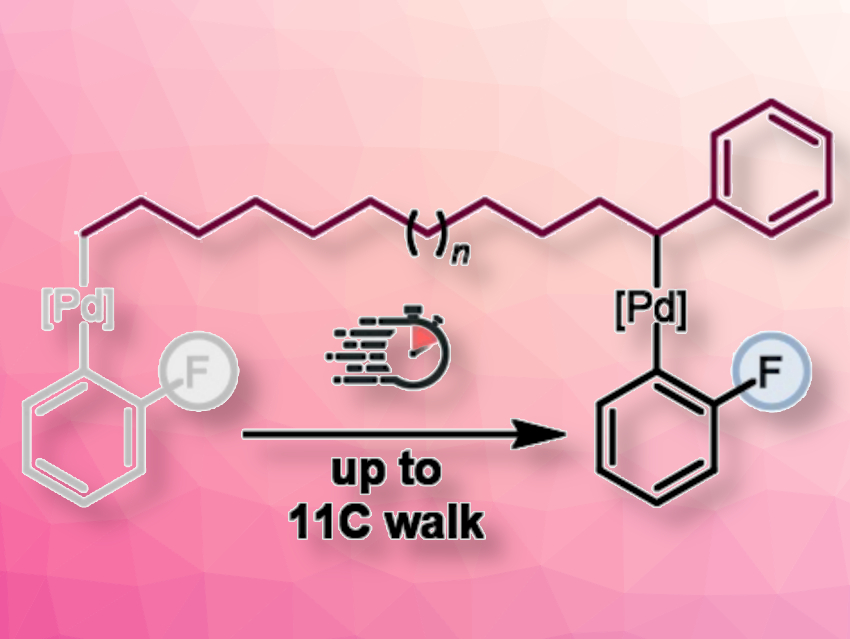
Franziska Schoenebeck, RWTH Aachen University, Germany, and colleagues have developed a rapid remote functionalization strategy to access ortho-fluorinated 1,1-diaryl alkanes with medicinal significance (pictured). In the presence of an air-stable Pd(I) dimer, [Pd(μ-I)(PCy2tBu)]2, the addition of arylalkyl-ZnCl to an N-methyl-2-pyrrolidone (NMP) solution of an ortho-fluoroaryl triflate generated valuable 1,1-diaryl alkane motifs. In contrast to these “chain-walking” reactions, the tri-tert-butylphosphine analogue of the Pd(I) dimer catalyst, [Pd(µ-I)PtBu3]2, triggered direct coupling at the initiation site.

The migratory coupling reaction occurs within 10 minutes at room temperature for chains with up to eleven carbon atoms. The reaction tolerates a wide range of functional groups. The potentially reactive ortho-fluoro and additional C–Cl sites in the substrates remained untouched, which can allow further diversification.
- Air‐Stable Pd(I) Dimer enabled Remote Functionalization: Access to Fluorinated 1,1‐Diaryl Alkanes with Unprecedented Speed,
Gourab Kundu, Filip Opincal, Theresa Sperger, Franziska Schoenebeck,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202113667