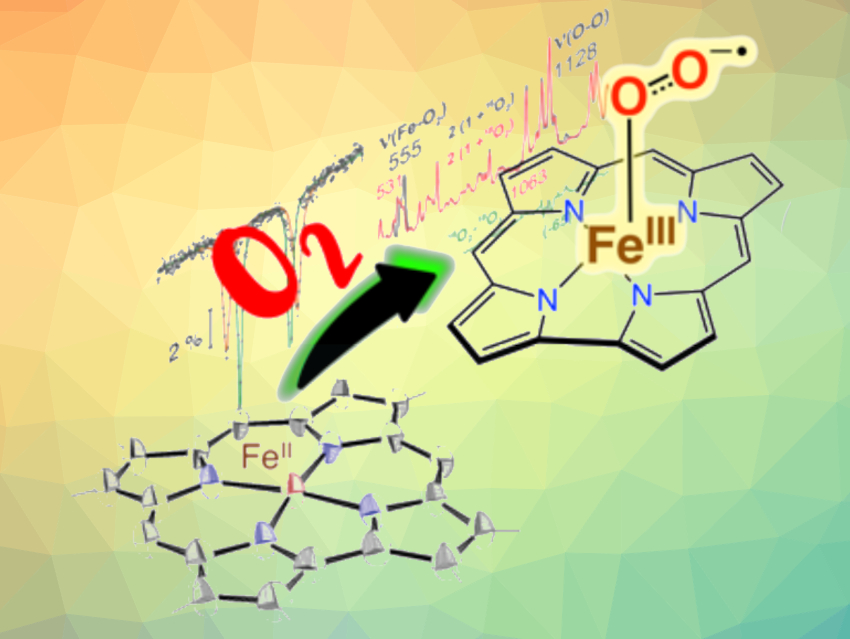
The utilization of dioxygen in nature is often mediated by the binding of O2 to transition metals, such as iron. These metals can then transfer multiple electrons to the bound O2. The transfer of one electron produces a metal superoxide species, which is proposed as a key intermediate in a number of oxidizing enzymes—including heme enzymes such as the tryptophan- and indoleamine-2,3-dioxygenases, and nonheme enzymes such as the thiol dioxygenases.
David P. Goldberg, Johns Hopkins University, Baltimore, MD, USA, Pierre Moënne-Loccoz, Oregon Health & Science University, Portland, OR, USA, and colleagues have found that an iron corrole compound can bind O2 to form an iron superoxide species at low temperature (pictured). Metallocorroles are versatile heme analogues, but until now, the canonical FeO2 adduct of a corrole had not been identified definitively.
The team reduced FeIII(ttppc) (ttppc = 5,10,15-tris((2,4,6-triphenyl)phenyl)corrolato3–), with sodium/mercury amalgam to obtain [FeII(ttppc)]−. A reaction with excess O2 at −80 °C then gave the superoxo complex [FeIII(O2−·)(ttppc)]−.
The new iron superoxide can carry out biologically relevant oxidations, such as indole dioxygenation and hydrogen-atom abstraction. The work provides insights into the reactivity of biomimetic iron superoxide species, as well as spectroscopic benchmarks for the future identification of such species.
- An Iron(III) Superoxide Corrole from Iron(II) and Dioxygen,
Jireh Joy D. Sacramento, Therese Albert, Maxime Siegler, Pierre Moënne‐Loccoz, David P. Goldberg,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202111492