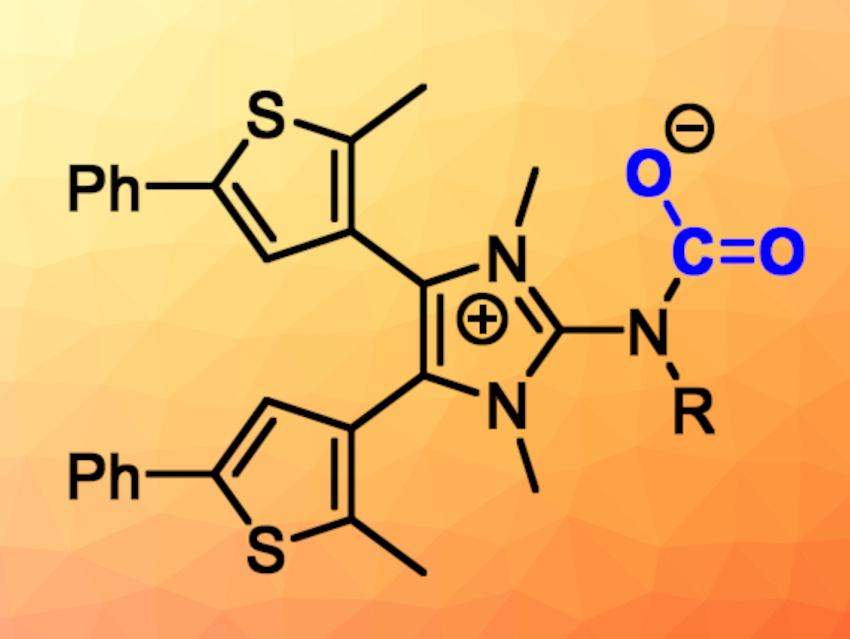
Superbases that can reversibly capture CO2 could provide a promising, low-energy method for the activation of the relatively inert CO2 molecule. The control of such chemical reactions with light is particularly appealing.
Frank Glorius, University of Münster, Germany, Fabian Dielmann, University of Münster and University of Innsbruck, Austria, and colleagues have developed a photoswitchable molecule that captures CO2 and releases it again upon exposure to UV light (pictured below). The process can be repeated after the regeneration of the superbase with visible light. The N-heterocyclic imine used for this was synthesized in seven steps starting from 2-methylthiophene. It undergoes a reversible, near quantitative isomerization upon successive exposure to UV and visible irradiation, which allows for the reversible CO2 capture.
.jpg)
The team found a remarkable difference of 8.7 pKa units for the basicity of the nitrogen atoms in the two isomeric forms, which exceeds the basicity changes of previously reported photoswitchable bases. This large difference in basicity, in combination with a nearly quantitative and repeatable switching behavior, could be helpful for the development of new photoswitchable transformations.
- Photoswitchable Nitrogen Superbases: Using Light for Reversible Carbon Dioxide Capture,
Lukas F. B. Wilm, Mowpriya Das, Daniel Janssen-Müller, Christian Mück-Lichtenfeld, Frank Glorius, Fabian Dielmann,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202112344