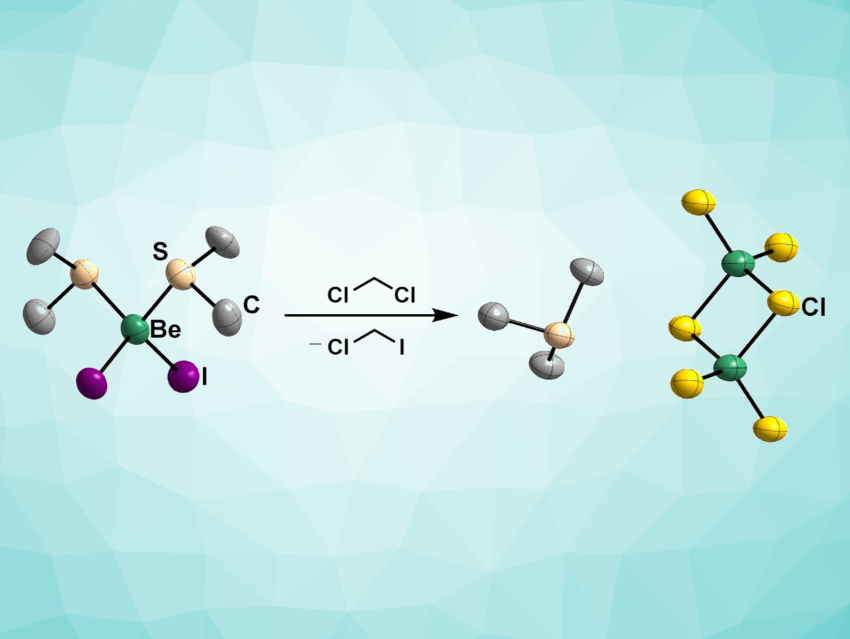
Inorganic beryllium chemistry currently has only a relatively low number of available starting materials. Beryllium halides are essential starting materials and can be readily prepared from their respective elements, but the insolubility of these compounds can hamper reactivity. Strong ligands such as ethers can provide soluble beryllium halide adducts. However, the ethers bind strongly to beryllium, which can be problematic. Thioether adducts with a comparatively weak Be–S bond strength could be more useful precursors, e.g., for beryllium alkyls.
Magnus Richard Buchner, University of Marburg, Germany, and colleagues have prepared thioether diadducts of beryllium halides, i.e., [(SMe2)2BeX2] (X = Cl, Br, I), which are remarkably soluble in non-polar solvents. The team reacted beryllium chloride, bromide, or iodide with dimethylsulfide to obtain the desired products. Adding another equivalent of the corresponding beryllium halide produced the μ2-halido-bridged monoadducts [(SMe2)BeX2]2. These compounds have improved solubility and the preparation is uncomplicated.
The S–Be interaction is weak, meaning that subsequent reactions of these compounds with other ligands should proceed unhindered, which makes them versatile starting materials. Even though the synthesized compounds decompose in solution, this process is slow enough to allow for further conversions.
- Dimethylsulfide Adducts of the Beryllium Halides,
Magnus Richard Buchner, Lewis R. Thomas-Hargreaves, Lukas K. Kreuzer, Nils Spang, Sergei I. Ivlev,
Eur. J. Inorg. Chem. 2021.
https://doi.org/10.1002/ejic.202100812