Silver(III) is the highest oxidation state currently available for the element. Ag(III) is a powerful oxidant with which a limited number of ligands are compatible. Simple compounds such as AgCl3, AgBr3, or AgI3 are still unknown, as are the corresponding [AgX4]− ions.
Babil Menjón, CSIC–Universidad de Zaragoza, Spain, and colleagues have isolated the mixed halide organocomplexes [(CF3)2AgCl2]– and [(CF3)2AgBr2]– as their trans isomers. The team added the homoleptic organosilver(I) compound [PPh4][CF3AgCF3] to a solution of Cl2 to obtain [PPh4][trans-(CF3)2AgCl2]. The bromo-derivative [PPh4][trans-(CF3)2AgBr2] was prepared via a reaction with Br2 following a similar procedure.
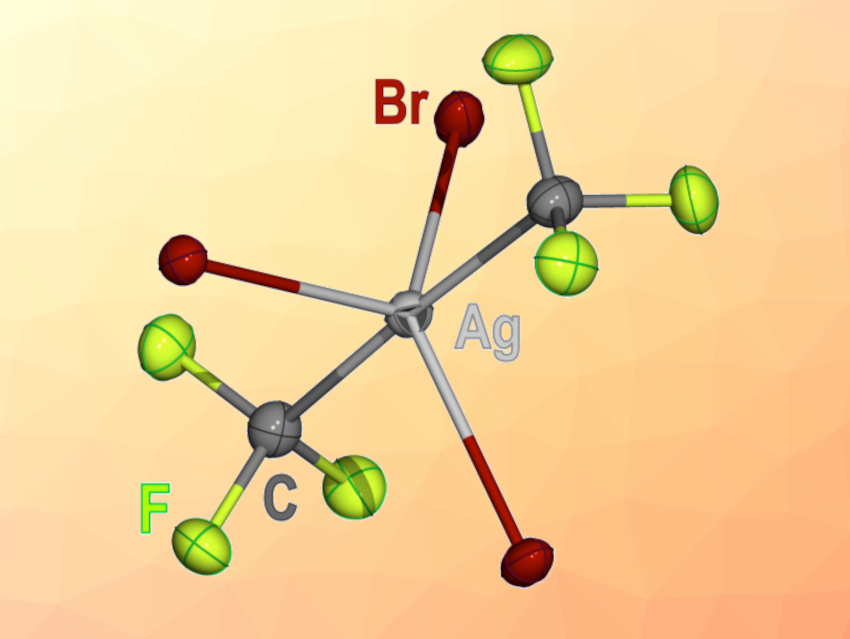
The resulting complexes are square-planar, and they are prone to ligand association to form complexes such as [PPh4]2[(CF3)2AgBr3] (anion pictured). The latter complex is unusual in that it is the first Ag(III) compound with trigonal symmetry and also the first five-coordinate compound with an inverted ligand field.
- The First Five‐coordinate Compound with Inverted Ligand Field: An Unprecedented Geometry for Silver(III),
Babil Menjón, Daniel Joven-Sancho, Miguel Baya, Antonio Martín, Jesús Orduna,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202112449