Axially chiral biaryls are widespread in natural products and pharmaceuticals. They are also widely used as chiral ligands in asymmetric catalysis. The chelation-assisted asymmetric C–H functionalization between arenes and electrophilic reagents, such as diazonaphthoquinones, provides an efficient way to obtain such scaffolds. However, this approach generally requires a preinstalled chelating group on the arenes and the addition of oxidants.
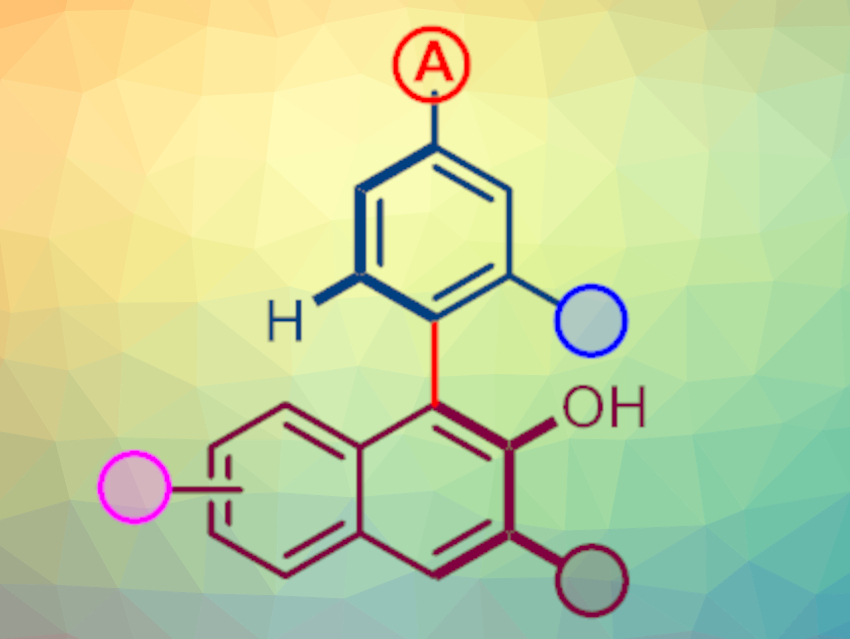
Jiangtao Sun, Changzhou University, China, and colleagues have developed an asymmetric C–H insertion reaction for the synthesis of axially chiral biaryls (pictured below) without the need for chelation groups or oxidants. The team used a chiral dirhodium(II) tetracarboxylate catalyst derived from N-1,2-naphthaloyl-(S)-tert-leucine (S-NTTL), Rh2(S-NTTL)4, to carry out an asymmetric insertion of rhodium carbene into the C(sp2)–H bond of arenes, followed by a point-to-axis chiral transfer. Using this catalyst, the researchers reacted a variety of 1-diazonaphthoquinones (pictured in dark red) with different aniline or anisole derivatives (pictured in blue).

The desired products were obtained in moderate to good yields with good enantioselectivities. The synthesized chiral biaryls can be used, e.g., to synthesize novel phosphine ligands.
- Construction of C‐C Axial Chirality via Asymmetric Carbene Insertion into Arene C‐H Bonds,
Ziyong Li, Ying Chen, Chuang Wang, Guangyang Xu, Ying Shao, Xinhao Zhang, Shengbiao Tang, Jiangtao Sun,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202110430