Polypropylene makes up ca. 30 % of all plastic waste. However, efforts to selectively convert polypropylene waste chemically have not been particularly successful. The chemical recycling of polypropylene usually yields a mix of solid, liquid, and gaseous compounds as cleavage products. This suggests that the design of catalysts that can control the selectivity of this process is challenging.
Javier Pérez-Ramírez, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have developed a carbon-supported platinum nanoparticle catalyst that can achieve complete hydrocracking of polypropylene into liquid hydrocarbons (C5–C45). The team used a surfactant-free colloidal synthesis to obtain monodisperse Pt nanoparticles. The nanoparticles were supported on a carbon material synthesized via the carbonization of an aniline/phytic-acid-based polymer.
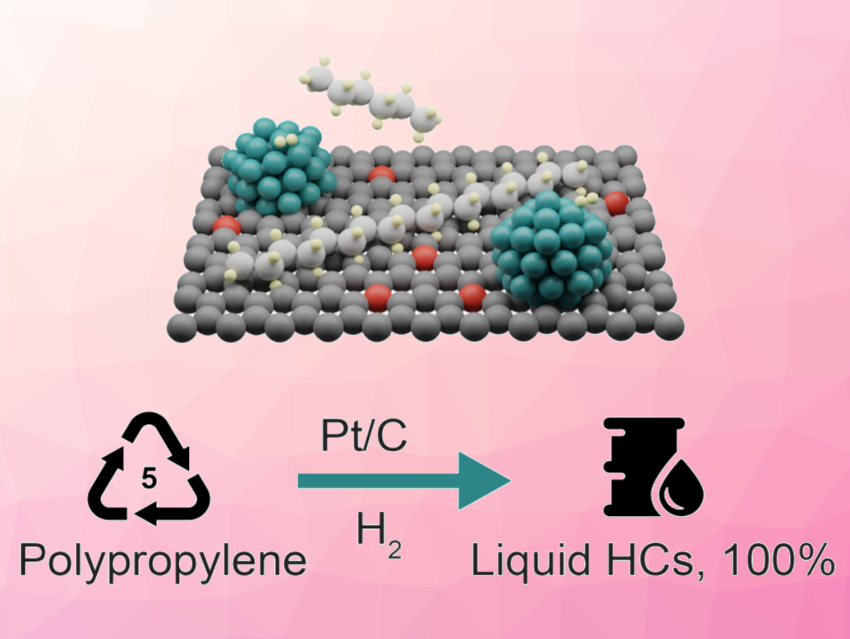
The team found that the metal and carrier phases (pictured) work together. The platinum phase controls the activity, while the carbon carrier regulates selectivity. The carrier readily adsorbs large polypropylene chains and desorbs shorter hydrocarbon products of specific lengths. The chain length at which products desorb can be tuned by changing the surface oxygen concentration on the carrier. Following this strategy, a selectivity of 80 % can be achieved toward motor oil (C21–C45). The work could provide some guidelines for catalyst design in the field of plastic hydrocracking.
- Direct Conversion of Polypropylene into Liquid Hydrocarbons on Carbon‐Supported Platinum Catalysts,
Shibashish D. Jaydev, Antonio J. Martin, Javier Pérez-Ramírez,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202101999