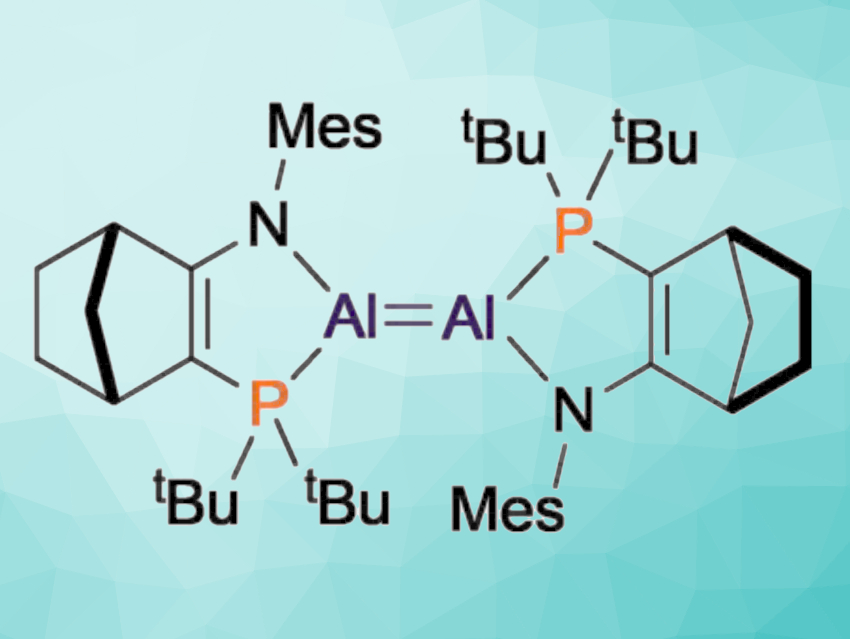
Dialumenes are multiply-bonded Al(I) compounds. Their high reactivity has attracted the attention of chemists because it enables, e.g., the bond activation of usually unreactive small molecules. However, the same high reactivity makes dialumenes challenging to isolate and study, which means relatively little is known about them.
Michael J. Cowley, University of Edinburgh, UK, Tobias Krämer, Maynooth University, Ireland, and colleagues have prepared a dialumene with a weak Al=Al bond that dissociates in solution. By reducing an Al(II) precursor with an Na/K alloy, an amido-phosphine-supported dialumene was prepared (pictured below). As a solid, the compound is Al=Al bonded, but in solution, the Al=Al bond can dissociate to generate Al(I) monomers.
.jpg)
Density functional theory (DFT) calculations showed that the Al=Al bond in the dialumene has only marginal multiple-bond character. The dialumene has a long Al=Al bond and a trans-bent structure. The researchers believe that understanding the effects of substituents at Al=Al bonds on the structure, bonding, and reactivity could enable the design of new dialumenes with controllable reactivity patterns.
- Reversible Dissociation of a Dialumene,
Rosalyn L. Falconer, Keelan M. Byrne, Gary S. Nichol, Tobias Krämer, Michael J. Cowley,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202111385