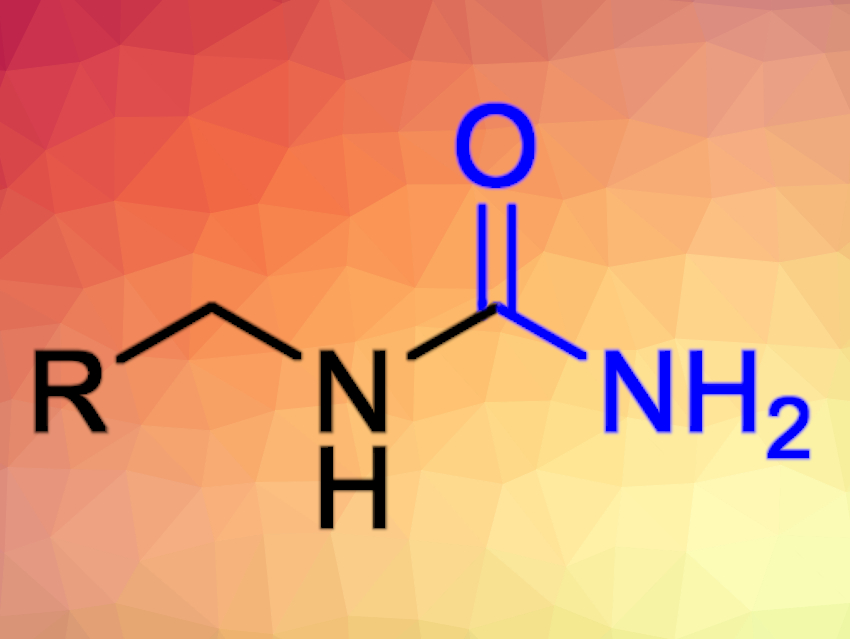
Monosubstituted ureas are important precursors to valuable chemicals such as drugs and herbicides. Existing syntheses for this type of compound generally require either toxic reagents (e.g., phosgene) or harsh conditions (e.g., heating in hydrochloric acid) and are, therefore, not compatible with many functional groups.
Karine Auclair and Chunling Blue Lan, McGill University, Montreal, Canada, have developed a simple, efficient method to synthesize monosubstituted ureas (pictured). The team used amines as substrates, potassium cyanate (KOCN) as a reagent, and ammonium chloride to promote the transformations. The reactions were performed using microwave irradiation at 120 °C in water.

The team obtained good to excellent yields within 15 min. The process tolerates a large scope of substrates (50 examples). It is compatible with acid-labile functionalities and common protecting groups. The team demonstrated the versatility and utility of their method by preparing 1-(2-fluoro-6-(trifluoromethyl)benzyl)urea, a key building block for the synthesis of elagolix, a drug used in the treatment of pain associated with endometriosis.
- Ammonium Chloride‐Promoted Rapid Synthesis of Monosubstituted Ureas under Microwave Irradiation,
Chunling Blue Lan, Karine Auclair,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202101059