Nitrites and nitrates are key chemicals, produced in large quantities for use as fertilizers and in the food and chemical industries. However, the current methods for their production generate significant quantities of greenhouse gases, most notably CO2 and N2O. The electrochemical oxidation of ammonia powered by renewable electricity could be a sustainable, low-temperature alternative for the decentralized production of these important compounds.
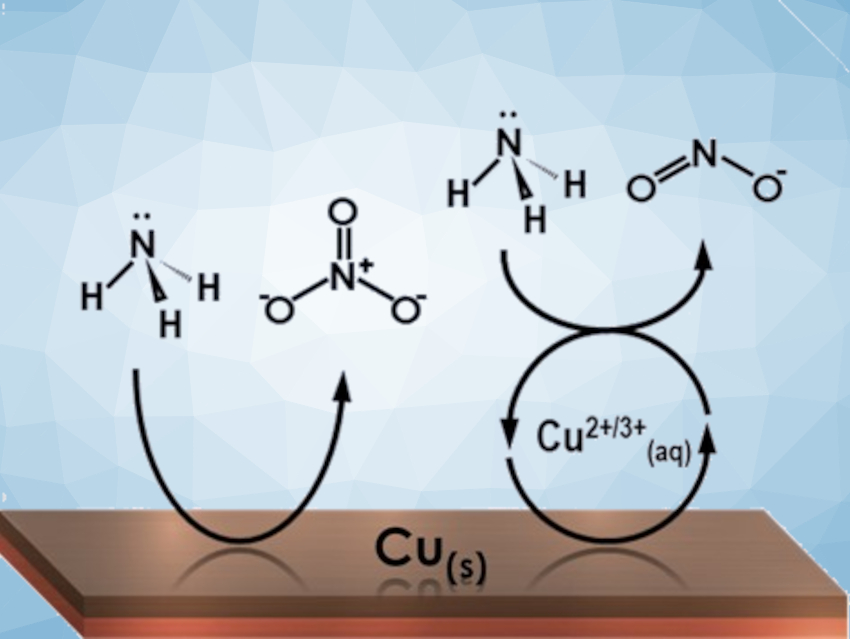
Alexandr N. Simonov, Bryan H. R. Suryanto, Monash University, Clayton, Australia, and colleagues have investigated the use of copper as an electrocatalyst for the conversion of ammonia to nitrite and nitrate. The team found that copper electrodes exhibit high electrocatalytic activity and selectivity for the oxidation of NH3 to NO2– and NO3– in alkaline solutions with a faradaic efficiency of up to ca. 86 %. The electrolyte solutions used contained KOH and NH4OH, and copper plates or wires were used as electrodes.
The selectivity between nitrate and nitrite can be switched by changing the mode of catalysis from homogeneous to heterogeneous (pictured) through tuning the pH and potential. The primary product of the heterogeneous pathway is NO3–. The primary product of the homogeneously-mediated reaction pathway, in which [Cu(OH)4]–/2– species dissolve from the electrode, is NO2–. The stable, continuous nature of the homogeneous catalytic reaction was demonstrated in week-long experiments.
- Copper‐catalysed electrosynthesis of nitrite and nitrate from ammonia: tuning the selectivity via an interplay between homogeneous and heterogeneous catalysis,
Sam Johnston, Liam Kemp, Bila Turay, Alexandr Nikolaevich Simonov, Bryan H. R. Suryanto, Douglas R. MacFarlane,
ChemSusChem 2021.
https://doi.org/10.1002/cssc.202101557