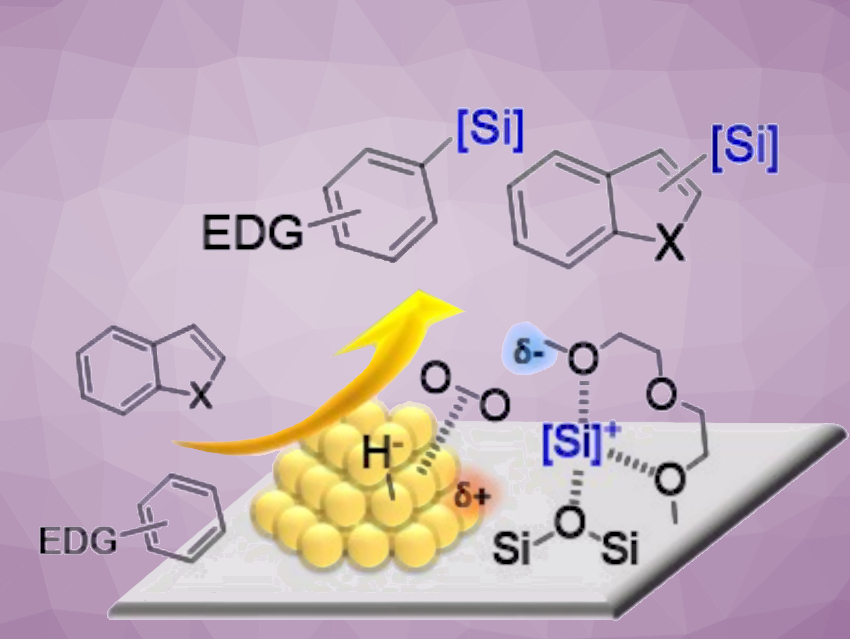
Aryl- and heteroarylsilanes have applications, e.g., in synthetic, pharmaceutical, and materials chemistry. Direct C(sp2)–H bond silylation with hydrosilanes is a straightforward and atom-economical approach for accessing aryl- and heteroarylsilanes. The only potential byproducts are nontoxic molecular hydrogen and water.
Hiroki Miura, Tetsuya Shishido, Tokyo Metropolitan University and Research Center for Hydrogen Energy-Based Society, Tokyo, Japan, Kyoto University, Japan, and colleagues have found that gold nanoparticles supported on silica show high catalytic activity for the direct silylation of C–H bonds in a variety of heteroarenes and electron-rich arenes to give the corresponding heteroaryl- and arylsilanes (pictured). The reaction was performed in the presence of an ether, such as bis(2-methoxyethyl) ether (diglyme), under an O2 atmosphere at ambient pressure. The desired heteroaryl- and arylsilanes were obtained in good to excellent yields.
The team attributed the efficient electrophilic C–Si bond formation to the generation of highly reactive silyl cations via heterolysis of the Si−H bond, cooperatively promoted by the ether and O2-activated gold nanoparticles. The supported gold catalyst could be reused repeatedly. These environmentally-friendly heterogeneous catalysts could enable the sustainable synthesis of aryl- and heteroarylsilanes.
- Electrophilic C(sp2)–H Silylation by Supported Gold Catalysts,
Hiroki Miura, Ryuji Hirata, Tomoya Toyomasu, Tetsuya Shishido,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202101123