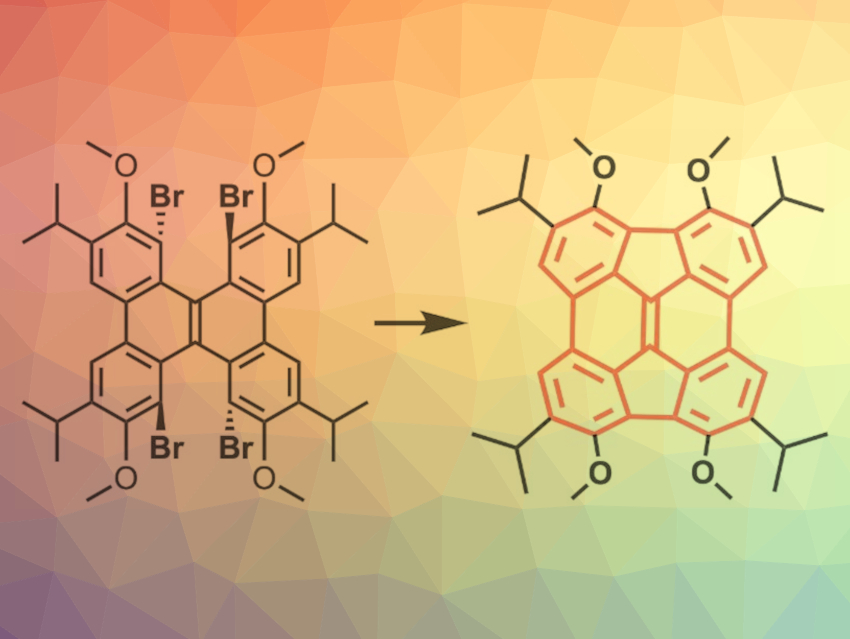
Diindeno(1,2,3,4-defg:1′,2′,3′,4′-mnop)chrysene (DIC, pictured in red) is one of the buckminsterfullerene fragments commonly referred to as “buckybowls”. Buckybowls have a curved π-surface, leading to unique optoelectronic properties. Other examples of buckybowls are, i.e., corannulenes and sumanenes. There are only a few known syntheses of DIC with no option of installing functional groups. Functionalizing DIC could improve its solubility and allow the optimization of the buckybowl’s properties for applications.
Tetsuo Iwasawa, Ryukoku University, Otsu, Japan, and colleagues have developed a solution-phase synthesis of multi-substituted DIC (pictured). The team used distorted dibenzo[g,p]chrysenes bearing four bromines, four methoxy ethers, and four alkyl groups (either iPr or tBu) as a precursor. Palladium-mediated ring-closing reactions then led to the desired buckybowls in yields up to 69 % for the iPr-substituted variant and up to 25 % for the tBu-substituted product.
The team performed the syntheses of both derivatives on a gram scale. Crystallographic analyses confirmed their bowl-like shapes. The researchers also demonstrated that the synthesized scaffold can be further transformed and functionalized, providing a basis for the continued exploration of this type of buckybowl.
- Solution‐Processable Multi‐Substituted Buckybowls: Synthesis of Diindeno(1,2,3,4‐defg:1′,2′,3′,4’‐mnop)chrysene Derivatives,
Naoki Yoshida, Ryuhei Akasaka, Yusuke Awakura, Toru Amaya, Tetsuo Iwasawa,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100869