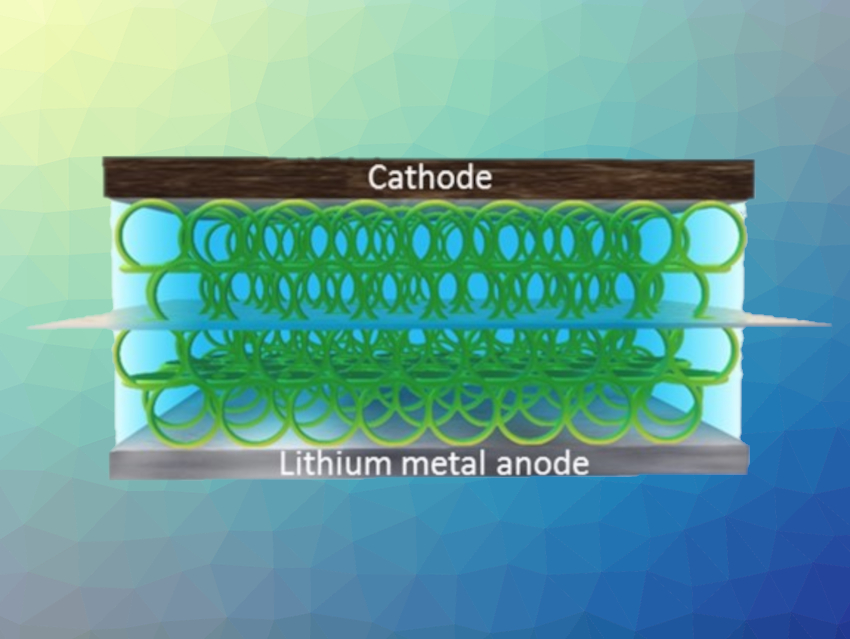
Lithium metal could be a useful alternative to traditional graphite-based anodes for the development of next-generation batteries due to its high specific capacity and low electrochemical potential. However, an unstable interface and potentially dangerous lithium-dendrite growth impede the practical use of lithium-metal batteries.
Ali Coskun, University of Fribourg, Switzerland, Jang Wook Choi, Seoul National University, South Korea, and colleagues have developed a gel-polymer electrolyte containing imidazolium ionic liquid end groups bearing fluorinated alkyl chains (pictured below in red). Ionic liquids are salts that are liquid at low temperatures.
The team prepared the electrolyte from the ionic liquid units bearing a fluorinated alkyl side chain (F-IL, pictured in red), dipentaerythritol penta- or hexaacrylate (pictured in black), and poly(ethylene glycol) methacrylate (PEGMA, pictured in blue). These monomers were polymerized using a free radical polymerization initiated by azobisisobutyronitrile (AIBN) in lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as an electrolyte and 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) as solvents with LiNO3 as an additive to give the desired gel.

The developed electrolyte provides a high lithium-ion conductivity and effectively suppresses lithium dendrite growth. The fluorinated ionic liquid promotes stronger anion coordination and weaker cation coordination, and thus, enables favorable lithium transport properties.
- Ionic Liquid Functionalized Gel Polymer Electrolytes for Stable Lithium Metal Batteries,
Tianhong Zhou, Yan Zhao, Jang Wook Choi, Ali Coskun,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202106237