CO2 is a greenhouse gas that plays a major role in climate change. Solving this environmental crisis has become an extremely urgent global issue. Converting CO2 into value-added chemicals by photocatalytic reduction is one strategy to alleviate CO2 pollution. As CO2 is very stable and chemically fairly inert, it requires appropriate catalysts to promote the reduction process.
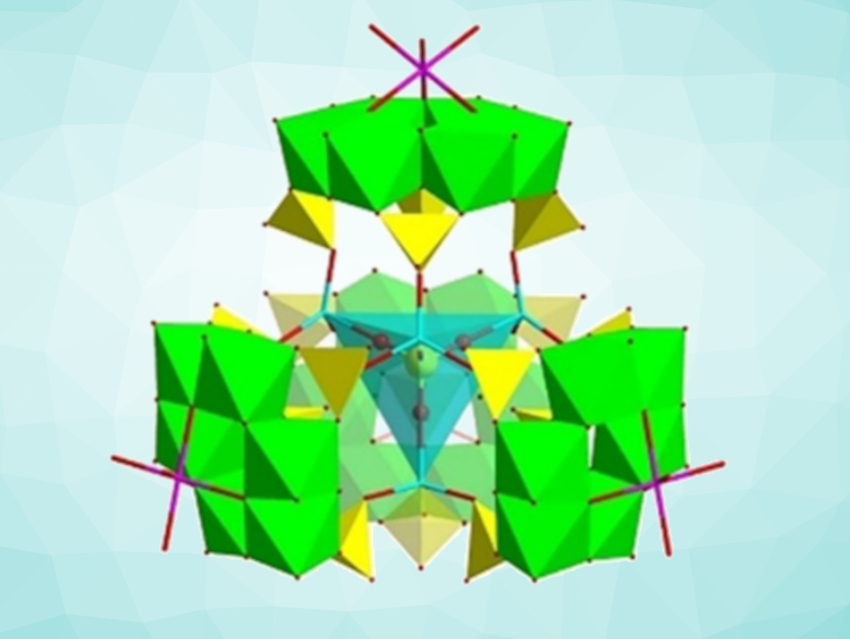
Yan Xu, Nanjing Tech University and Nanjing University, China, and colleagues have synthesized the purely inorganic, polyoxometalate (POM)-type cluster H47Na2Co4Mo24(PO4)11O72⋅15 H2O (or {Co4Mo24}, pictured) that shows photocatalytic activity for the conversion of CO2 to CO. The team prepared the cluster via a hydrothermal synthesis. The cluster has a “quasi-Keggin” structure and can be thought of as an assembly of a central {Co2PO4} tetrahedron and four peripheral {Co[P4Mo6]} fragments, resulting in the generation of a 3D framework with large cavities. The catalyst, thus, features a combination of reductive {P4Mo6} units and Co active centers.
Under visible-light irradiation, the photocatalytic system provides a CO formation rate of 1848.3 μmol g–1 h–1 and a high selectivity value of 97.0 %, with excellent CO evolution stability over four cycles. The work could serve as a framework for the design of new polyoxometalate-based materials for CO2 reduction.
- A Purely Inorganic Quasi-Keggin Polyoxometalate for Photocatalytic Conversion of Carbon Dioxide to Carbon Monoxide,
Xiao-Mei Liu, Run-Kun Kang, Ji-Lei Wang, Jia-Nian Li, Qiao-Ling Chen, Yan Xu,
ChemPlusChem 2021.
https://doi.org/10.1002/cplu.202100260