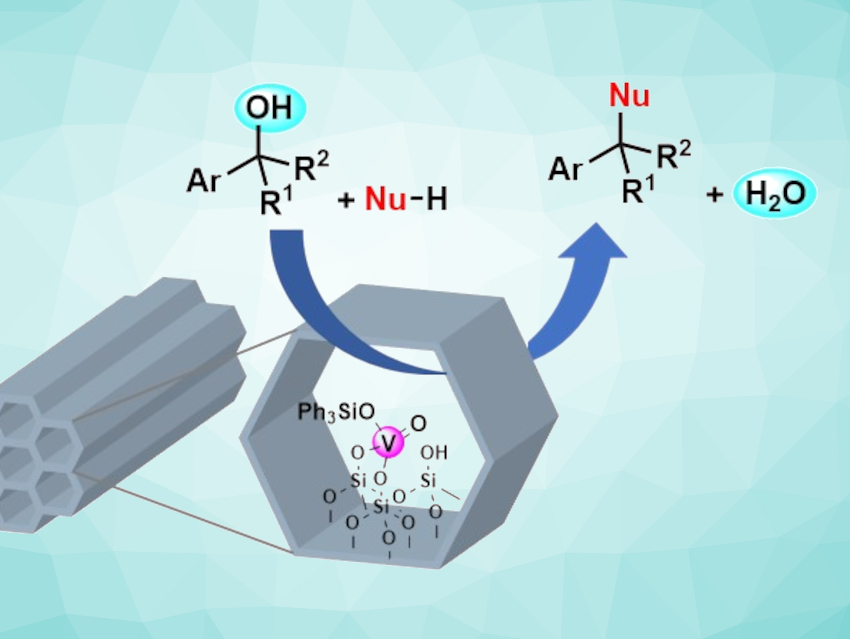
The direct substitution of alcohols by nucleophiles is useful in green chemistry because it generates water as the only byproduct. Some Lewis acid-catalyzed direct substitution methods have been reported, but they often suffer from poor chemoselectivity.
Shuji Akai, Osaka University, Japan, and colleagues have developed an operationally simple, highly chemoselective protocol for nucleophilic substitution reactions of alcohols (pictured). The team used a mesoporous silica (MPS)-immobilized oxovanadium catalyst (VMPS4, pictured schematically) to react different thiols or carbon nucleophiles with benzyl or allyl alcohols. The catalyst was prepared by adding VO(OSiPh3)3 to mesoporous silica.
The team found that VMPS4 shows excellent selectivity in a competitive reaction of alcohols and acetates with dodecanethiol as the nucleophile: The alcohol was quantitatively transformed into the corresponding thioether, while the acetate remained intact. This result was significant because the acetoxy group is generally a better leaving group than the hydroxyl group. Other common Lewis acid catalysts resulted in poor chemoselectivity for the same competitive reaction. The VMPS4-catalyzed direct substitution reaction offers a broad substrate scope, and the catalyst can be recovered and reused up to seven times.
- Direct nucleophilic substitution of alcohols using an immobilized oxovanadium catalyst,
Tomoya Nishio, Shin Yoshioka, Kai Hasegawa, Kenzo Yahata, Kyohei Kanomata, Shuji Akai,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100569