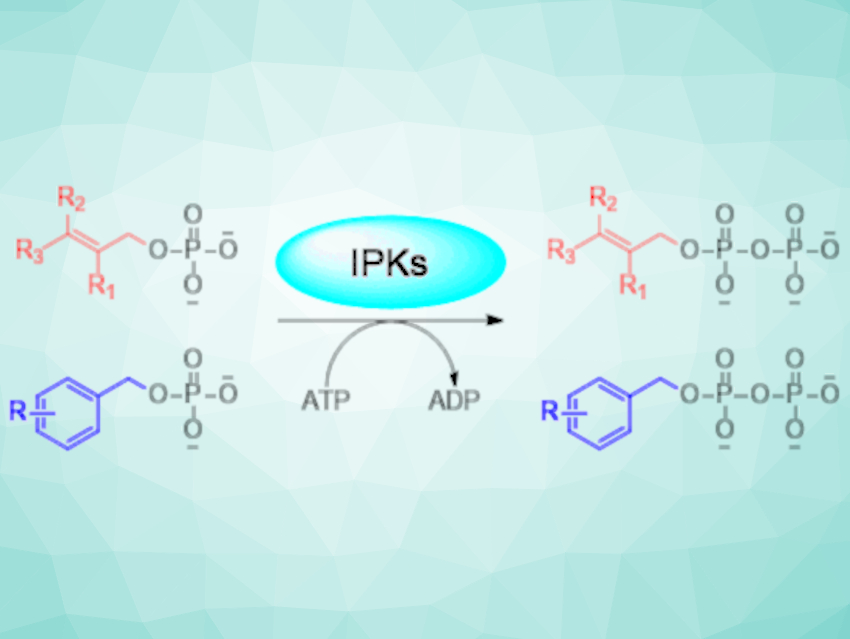
Isoprenoids are a diverse class of natural products with a range of applications in, e.g., medicine or as biofuels. There have been significant research efforts to develop new pathways for the large-scale production of valuable isoprenoids, e.g., using enzymes. Cascade enzyme reactions can be used to enhance the range of suitable substrates and develop new compounds. For example, alcohol kinase and isopentenyl phosphate kinase (IPK) cascades can be used to generate diphosphate precursors from isoprene alcohols. However, the breadth and depth of the substrate promiscuity of IPKs as an enzyme class remains underexplored.
Shanteri Singh, University of Oklahoma, Norman, USA, and colleagues have investigated the substrate breadth of five novel IPKs from various Archaea (single-celled organisms). The team used a library of 58 synthetic alkyl-monophosphate analogs (examples pictured below on the left) and found that 38 of them were viable substrates. Then, they coupled the IPK-catalyzed reaction of these substrates with an aromatic prenyltransferase (FgaPT2) to obtain alkyl-functionalized L-tryptophan derivatives (pictured below on the left).

This work demonstrates the utility of IPKs as biocatalytic tools for the synthesis of non-natural isoprenoids and shows that IPK-catalyzed reactions are compatible with downstream isoprenoid enzymes.
- Novel Homologs of Isopentenyl Phosphate Kinase Reveal Class-Wide Substrate Flexibility,
Vikas Kumar, Bryce P. Johnson, Dustin A. Dimas, Shanteri Singh,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202100595