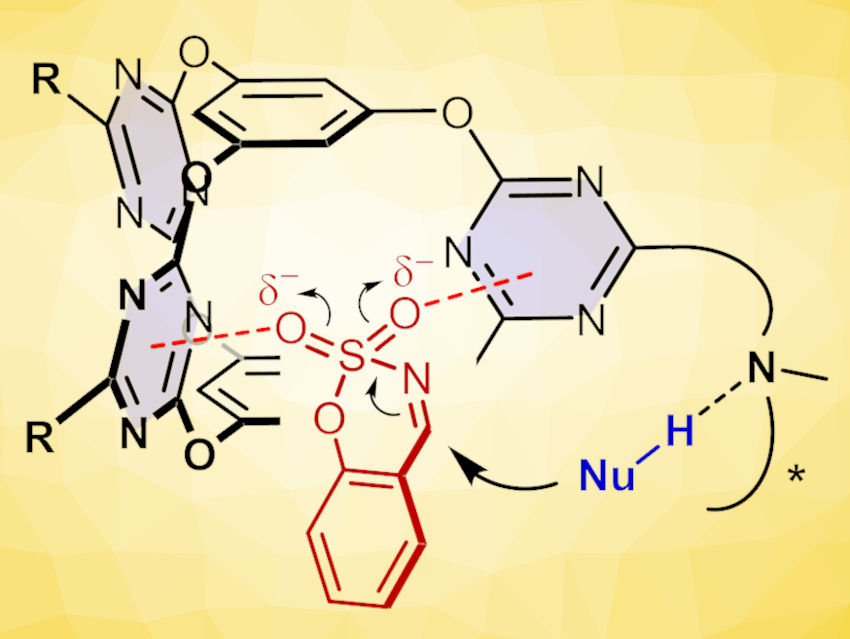
Anion-π interaction, i.e., the attraction between an anion and an electron-deficient aromatic ring, can drive catalysis, e.g., by stabilizing an anionic reaction transition state. In contrast to conventional “site-activation”, this “π-face activation” is rather flexible. This can be a significant advantage, but effective activation and selectivity control are still challenging.
Qi-Qiang Wang, Beijing National Laboratory for Molecular Sciences, Chinese Academy of Sciences, and University of the Chinese Academy of Sciences, Beijing, and colleagues have developed a catalysis strategy based on cooperative anion-π activation using molecular cage catalysts with an electron-deficient π-cavity (pictured). The team functionalized a known bis(tetraoxacalix[2]arene[2]triazine) cage (pictured below on the left) with different chiral amines, including cyclohexanediamine, binaphthyldiamine, and cinchona alkaloids, in the presence of N,N-diisopropylethylamine (DIPEA) to obtain the desired catalysts (pictured below on the right).

The catalyst was then used in decarboxylative Mannich reactions. Just 2 mol % of the cage catalyst efficiently catalyzed decarboxylate Mannich reactions of sulfamate-headed cyclic aldimines (pictured at the top) with different malonic acid half thioesters in nearly quantitative yields and with excellent enantioselectivities. This work demonstrates that cooperative anion-π interactions within a confined environment can drive efficient and highly selective transformations. This could be useful for the development of other catalyst designs and applications based on the anion-π activation principle.
- Exploiting Anion-π Interactions for Efficient and Selective Catalysis with Chiral Molecular Cages,
Na Luo, Yu-Fei Ao, De-Xian Wang, Qi-Qiang Wang,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202106509