Efficient energy storage can be a challenge at high latitudes and altitudes, where temperatures are low. Lithium-ion batteries, for example, have limited capacity in the cold and are prone to failure because of the deposition of metallic Li. Therefore, new battery materials for low-temperature energy storage are needed.
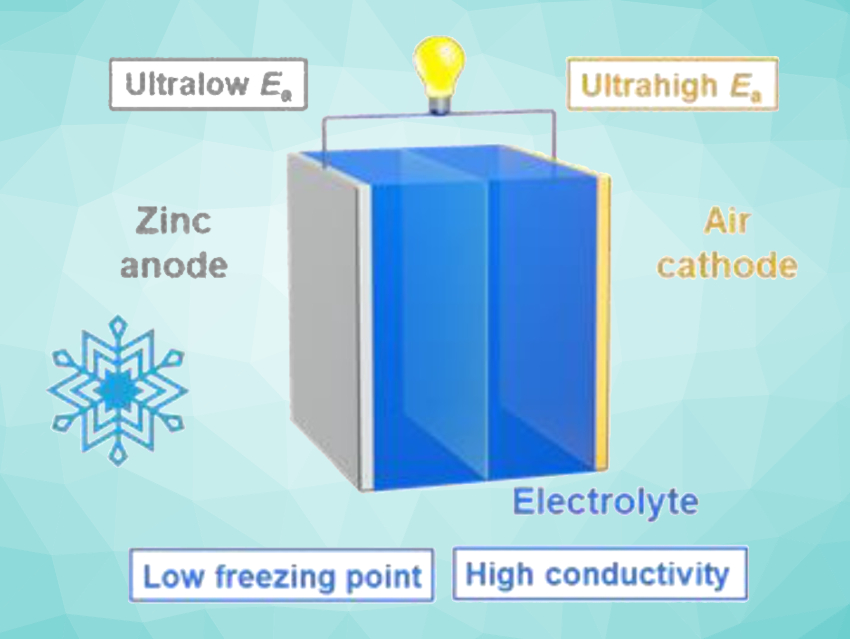
Bo-Quan Li, Beijing Institute of Technology, China, Qiang Zhang, Tsinghua University, Beijing, China, and colleagues have systematically investigated the low-temperature performances of zinc–air batteries (ZABs). The team found a –40°C working temperature for ZABs with a combined Pt/C and Ir/C cathodic electrocatalyst and an aqueous electrolyte containing aqueous KOH and Zn(OAc)2. Maximum output power densities of 29.9, 17.2, and 6.5 mW cm−2 were achieved at −20 °C, −30 °C, and −40 °C, respectively. This confirmed the working feasibility of ZAB at low temperatures.
However, the reduced ionic conductivity of the electrolyte and the resulting increased internal resistance in the battery were limiting factors. The researchers tested a range of alkaline electrolytes and found that a 6.0 M CsOH and 0.20 M Zn(OAc)2 mixture provides hydrogen bonding between hydroxide anions and water molecules for improved conductivity. The ZAB with the CsOH-based electrolyte was stable for 500 cycles with a voltage gap of 0.8 V at 5.0 mA cm–2 at –10 °C. The work shows the potential of zinc–air batteries for low-temperature electrochemical energy storage and could inspire advanced battery systems for use under extreme working conditions.
- Can Aqueous Zinc–Air Batteries Work at Sub-Zero Temperatures?,
Chang-Xin Zhao, Jia-Ning Liu, Nan Yao, Juan Wang, Ding Ren, Xiang Chen, Bo-Quan Li, Qiang Zhang,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202104171